Protocol/ Open Access
DOI: 10.31488 /bjhd.114
Negative-Pressure Therapy versus Hydrocolloid Dressing on the Prevention of Surgical Wound Infections in Cardiac Surgery: A Randomised Controlled Trial Protocol
Pilar Garrido-Martín1, Patricia Rodríguez-Fortúnez2, Javier Montoto-López1, Pablo Prada-Arrondo1, Guadalupe Sauchelli-Faas1, Paula Masiero-Aparicio2, Mónica García-Bouza1, Yakir Castillo-Eyzaguirre1, Celia Miranda-Barrero1, Soraya Stella-Gónzalez1, Susana Aceituno3, Estefanía Pérez-Carreño2, Rafael Martínez-Sanz1
1.Servicio de Cirugía Cardiaca. Hospital Universitario de Canarias. Tenerife. Spain
2.Unidad de Ensayos Clínicos. Servicio de Farmacología Clínica. Hospital Universitario de Canarias. Tenerife. Spain
3.Outcomes’10. Castellón de la Plana. Spain
*Corresponding author: Pilar Garrido-Martín PhD, Servicio de Cirugía Cardiovascular. Hospital Universitario de Canarias, Spain.
Abstract
Negative-pressure wound therapy (NPWT) and hydrocolloid dressings have been shown to reduce surgical wound infections (SWI) after cardiac surgery. However, the comparative effectiveness of these two alternative dressings has not been evaluated to date. This study describes a randomised controlled trial protocol to evaluate the efficacy of using portable single-use NPWT versus single-use hydrocolloid dressings in the prevention of SWIs in cardiac surgery under extracorporeal circulation. A randomised clinical trial for post-market surveillance of medical devices, with two-arm parallel groups, will be conducted in patients undertaking median sternotomy. The primary outcome to be assessed will be the prevention of SWIs, while the secondary outcome will be the economic cost. This study will determine whether NPWT can significantly reduce SWIs compared to hydrocolloid dressings, cost-effectively. This trial has been registered in ClinicalTrials.gov under the identifier NCT04265612 and approved by the Research Ethics Committee for medicinal products at the Hospital Universitario de Canarias.
Keywords: negative pressure wound therapy, hydrocolloid dressing, cardiovascular surgery, cardio-surgical infection prevention, sternotomy
Introduction
Surgical wound infections (SWI) are rare complications (3-6%) after open-heart surgery, but can have serious consequences and economic repercussions due to prolonged post-surgical stays [1]. Despite the use of draping techniques, meticulous surgical techniques, and skin preparation with ethanol/chlorhexidine, the surgical wound is still colonised by pathogens in many patients during cardiac surgery [2]. The most common surgical site infections (SSI) are of the sternotomy wound and the leg from which the vein was harvested for coronary bypass [3]. As contamination mainly occurs during surgery and early wound healing, most cases of SSI are observed in the first days or weeks after the initial surgical procedure, more specifically during the first days of the stay in the intensive care unit (ICU) [4].
Sternal wound infections can be classified as superficial or deep, based on the depth of the condition in the wound [5]. The incidence of superficial infections, such as SSI or dehiscence, ranges from 0.3% to 10%. Among deep wound infections, mediastinitis is the most severe form, occurring in 1-6% of cases, with morbidity of up to 50%, leading to a prolonged hospital stay and a mortality rate of 14-47% [6,7]. Once SWIs are diagnosed, they are initially treated with intravenous antibiotics. In additional to antimicrobial therapy, surgical treatment is generally required for deeper sternal SWI [8]. These complications are associated with
reduced quality of life for patients and increased social and health-care costs [9,10].
Traditionally, the surgical incision was covered with a braided gauze dressing that required changing, as well as surgical-wound cleaning, every 24 hours. Hydrocolloid dressings have subsequently been adopted in surgical practice to provide better wound healing conditions by maintaining a moist environment, gaseous exchange, and protection from secondary infection [11,12]. Fibrous hydrocolloid dressings are composed of sodium carboxymethylcellulose, which forms a gel when it comes into contact with fluid and prevents its propagation. Daily dressing changes are not necessary with fibrous hydrocolloid dressings, which can remain on the cardiac surgery wound for seven days. Their use has been to shown to prevent SSI of cardiac surgery and to be cost-effective [13].
Negative-pressure wound therapy (NPWT) is an alternative form of dressing, which can be applied to closed surgical incisions. In this treatment, a solid foam layer overlies the incision and is covered with a semipermeable membrane, which is only permeable to gas. A sealed tube is used to connect the foam with a pump, which creates a partial vacuum over the wound. This negative-pressure therapy provides a sealed environment, preventing bacterial ingress and removing blood and serous fluid exuding from the wound [14,15]. The application of NPWT has been shown to reduce wound complications such as infection, seroma or dehiscence after cardiac surgery, while also shortening the hospital stay compared to traditional therapy [16-18].
Notwithstanding the above, to the best of our knowledge, comparative studies have not been conducted on the effectiveness of NPWT versus hydrocolloid dressings. Therefore, the present work describes a randomised controlled trial (RCT) protocol to evaluate the efficacy of using portable single-use negative-pressure therapy versus single-use hydrocolloid dressings in the prevention of SWIs in cardiac surgery under extracorporeal circulation.
Materials and Methods
Protocol and registration
This trial has been approved by the Research Ethics Committee for medicinal products at the HUC (Hospital Universitario de Canarias) with internal registration number 06/19. The methodology is specified in advance, documented in a protocol and has been registered before beginning recruitment (ClinicalTrials.gov Identifier: NCT04265612) [19].
Design and setting
A randomised clinical trial of post-market surveillance of medical devices, with two-armed parallel groups, will be conducted (Figure 1).
Eligibility criteria for patients
All patients over 18 years of age subjected to elective or urgent cardiovascular surgery under extracorporeal circulation that are to undergo a median sternotomy, will be informed of the study and invited to participate. Patients will be assessed for eligibility by their primary surgeon. Exclusion criteria include urgent surgeries that do not allow time for randomisation and/or coding, patients with immunosuppressed haematological diseases, allergic or hypersensitive to the dressing or excipient, patients who due to their fragility or comorbidity are not considered eligible by the surgeon, or patients who are participating in another experimental study. Signed informed consent will be sought from all patients who meet all the criteria for inclusion and none for exclusion.
Interventions
Participants randomised to the experimental arm will have a PICO® negative pressure dressing (PICO, Smith & Nephew). The participants randomised to the active comparator group will have a hydrocolloid dressing: Aquacel Surgical® hydrogel (Aquacel Surgical, ConvaTec Inc.). In both groups, the dressings (PICO or Aquacel Surgical) will be placed on both sternal wound and saphenectomy in the operating room, and they will remain in place for seven days without removal, unless saturated, in which case, only the dressing will be changed.
Primary endpoint
The primary endpoint will be the incidence of infection of the sternal surgical wound. The state of the surgical wound will be assessed by surgeons and nurses, and recorded in the log for wound data collection. They will record whether the wound has a good appearance or not, and whether there are any complications: exudate (and its appearance), necrosis of the wound edges, redness, pain, maceration of the wound or wound dehiscence.
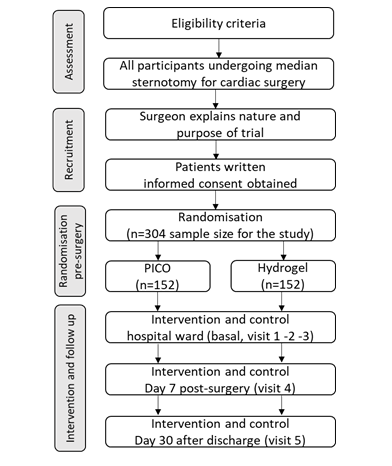
Figure 1:Flow diagram of randomisation procedure
Secondary endpoints
A secondary endpoint of the study is to compare whether there are differences between the application of PICO® dressing compared to Aquacel® Surgical single-use hydrocolloid dressings in terms of hospitalization length and hospital costs. For this purpose, days of hospitalisation in the ICU and in-ward during the postoperative period will be recorded. Subsequently, hospital costs (euros), derived from the surgical procedure, length of the hospital stay and the price of the products, will be calculated.
Randomisation
Each patient will be randomly assigned to one of the two treatment groups using a computerised sequence of randomised numbers. The series will be developed using computer-generated random numbers using Microsoft Excel software Microsoft Excel software 2013 (15.0.5311.1000) MSO (15.0.5311.1000) 32-bit. Participants will be assigned to either the experimental or comparator group in chronological order (date of inclusion) following a 1:1 block randomisation so that an equal number of patients will be allocated to each group. Each participant will be given a number when they are included in the study. Reoperations for bleeding do not apply to this study.
Sample size
Existing data from the register issued by the Preventive Medicine Service of the HUC indicate that the incidence rate of nosocomial infections of the sternal surgical wound in cardiac surgery in HUC was 5.8% in 2018 and 2019. It is expected that the incidence rate of nosocomial infections can be reduced to 1% using the intervention, and a sample size of n = 152 in each group (experimental or comparator) will be required to detect a difference of this magnitude with 80% power and α = 0.05. That means a final sample size for the study of n = 304 and an estimated study duration of about 18 months.
To estimate the number of patients required achieving significant differences between groups, with 80% power and a 5% type I error, an intermediate analysis will be carried out after including 60 patients (30 per group). Losses of up to 20% will be assumed in each group. Nevertheless, a statistical analysis will be carried out by protocol and by intention to treat (ITT).
Data collection and monitoring
Data for the study will be collected and recorded on data collection logs in hard copy format and subsequently entered into a database by a single researcher authorised for that purpose. Monitoring will be performed to check completeness and data queries.
Demographic and patient-related characteristics (age, weight, height, cardiovascular risk factors, coronary disease, catheterization, and comorbidities) will be collected during the basal visit, as well as the preoperative blood test results (on admission). Intraoperative data (visit 1) will include surgical data (coronary and/or valve surgery, surgical times, type of suture, characteristics of the surrounding skin, etc.) and the occurrence of adverse events (AE) will also be recorded (related with the dressing or not; serious or not). All study participants will be followed up postoperatively on days 2 (visit 2, ICU stay), 3-5 (visit 3, ward stay), 7 (visit 4, hospital discharge) and 30 (visit 5, after hospital discharge) for surgical wound assessment (wound condition and skin characteristics), blood test results and AEs (including possible complications during hospitalization or after discharge).
The appearance and possible change of the dressing will be recorded during visit 2, visit 3 and visit 4 in the section of the form corresponding to dressing assessment. Data collection is summarised in Table 1.
Table 1:Schedule of variables to collect during the study
| Procedures | Basal visit hospitalisation | Visit 1 surgery | Visit 2 ICU stay | Visit 3 Ward stay | Visit 4 Hospital discharge | Visit 5 30d after Hospital discharge |
|---|---|---|---|---|---|---|
| Inclusion/ Exclusion criteria | X | |||||
| Informed consent | X | |||||
| Blood analysis | X | X | X | X | X | |
| Demographic data and characteristics | X | |||||
| Surgical data | X | |||||
| Adverse events | X | X | X | X | X1 | |
| Surgical wound assessment1 | X | X | X | X | X | |
| Dressing assessment | X2 | X3 | X3 | X3 |
1The form to be filled in about the appearance of the wound.
2Placement of the dressing by the nurse and supervised by the surgeon.
3Assessment of the dressing. If a dressing needs to be changed, it can be done
either on the 1st day or during the week, but only up to a maximum of one
dressing change within seven days of the first application. If the PICO dressing
has already been changed once due to saturation and, on the same day, it
becomes saturated again, its application would be suspended as a preventive
measure, and the wound would be assessed, recording this as a complication.
Samples for microbiological culture will be taken if there is exudate or the
wound does not have a good appearance when dressings are removed.
Members from the Ethics Committee may follow up the protocol through face-to-face meetings. Moreover, the study may be audited by an independent board.
Adverse event management
All adverse events will be immediately referred to the surgeon, and appropriate actions will be taken. The PI (principal investigator) of the study will enter any adverse event on a normalised report form and will report them to the medical device vigilance point. Some adverse events, such as wound exudate, skin or sternal dehiscence and positive microbiological culture, are foreseeable as part of the proposed treatment. No systemic adverse reactions are expected. All participants experiencing adverse events will be followed up until the end of the trial.
Data analysis
All statistical analyses will be conducted using the Statistical Package for the Social Sciences (SPSS; version 17, Chicago, IL). Categorical variables will be presented as frequencies and percentages. Normally distributed continuous variables will be expressed as mean values and standard deviation. Those variables that do not follow a normal distribution will be presented as medians and interquartile ranges. Univariate comparisons will be made using either chi-square statistics or t-test/non-parametric test as appropriate. The inter-group difference in the speed of adverse events onset will be evaluated through the log-rank test. In addition to an analysis per protocol, all data will be analysed using an ITT strategy, so that patients will be in their allocated treatment group, regardless of the actual treatment received. All tests will be two-tailed and, following convention, a p-value < 0.05 will be taken to indicate a statistically significant association in all tests.
The economic evaluation will be carried out through a cost-effectiveness analysis that will be carried out using Microsoft Excel software 2013 (15.0.5311.1000) MSO (15.0.5311.1000) 32-bit. The probabilities will be obtained from the HUC data and by using the statistical package Stata version 13.0 (StataCorp LP, USA).
Discussion
Despite the number of publications demonstrating the efficacy of NPWT in reducing SWI compared to traditional bandages [20], this difference has not been demonstrated with respect to hydrocolloid dressings. Aquacel Surgical® hydrogel dressing was first introduced for non-infected open-chest management after cardiac surgery at the HUC in 2017. After adopting this therapy, the number of SWI in 2018 was reduced by 5.8%. In order to decrease this percentage even further, the PICO® negative pressure dressing was introduced in late 2018. Both therapies (PICO and Aquacel Surgical) are currently authorised by the HUC and are routinely used at the surgeon´s discretion. Their rate of application is approximately 80% for Aquacel Surgical and 20% for PICO. However, differences in effectiveness have not been evaluated. The present RCT is the first to compare NPWT versus hydrocolloid dressings in terms of prophylactic efficacy in the prevention of SWI after cardiac surgery and the associated costs.
Abbreviations
HUC: Hospital Universitario de Canarias, ICU: Intensive Care Unit, ITT: Intention to Treat, NPWT: Negative-Pressure Wound Therapy, RCT: Randomised Controlled Trial, SSI: Surgical Site Infections, SWI: Surgical Wound Infections
Acknowledgments
Our team would like to thank the Clinical Trials Unit of the Hospital Universitario de Canarias-SCReN Platform (Clinical Pharmacology Service) for their collaboration in the execution of the trial. We also thank the participating patients for their generosity and altruism.
Conflict of Interest
No conflict of interest was declared.
References
1. Vos RJ, Van Putte BP, Kloppenburg GTL. Prevention of deep sternal wound infection in cardiac surgery: a literature review. J Hosp Infect. 2018;100(4):411-420.
2. Kühme T, Isaksson B, Dahlin LG. Wound contamination in cardiac surgery: A systematic quantitative and qualitative study of bacterial growth in sternal wounds in cardiac surgery patients. APMIS. 2007;115(9):1001-1007.
3. Gudbjartsson T, Jeppsson A, Sjögren J, et al. Sternal wound infections following open heart surgery–a review. Scand Cardiovasc J. 2016;50(5-6):341-348.
4. Mekontso Dessap A, Vivier E, Girou E, et al. Effect of time to onset on clinical features and prognosis of post-sternotomy mediastinitis. Clin Microbiol Infect. 2011;17(2):292-299.
5. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309-332.
6. Ridderstolpe L, Gill H, Granfeldt H, et al. Superficial and deep sternal wound complications: Incidence, risk factors and mortality. Eur J Cardio-thoracic Surg. 2001;20(6):1168-1175.
7.Careaga Reyna G, Aguirre Baca GG, Medina Concebida LE, et al. Factores de riesgo para mediastinitis y dehiscencia esternal después de cirugía cardíaca. Rev Esp Cardiol. 2006;59(2):130-135.
8. Phoon PHY, Hwang NC. Deep sternal wound infection: diagnosis, treatment and prevention. J Cardiothorac Vasc Anesth. 2020;34(6):1602-1613.
9. Jidéus L, Liss A, Ståhle E. Patients with sternal wound infection after cardiac surgery do not improve their quality of life. Scand Cardiovasc J. 2009;43(3):194-200.
10. Mehaffey JH, Hawkins RB, Byler M, et al. Cost of individual complications following coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2018;155(3):875-882.e1.
11. Hermans MHE. Clinical benefit of a hydrocolloid dressing in closed surgical wounds. J Wound, Ostomy Cont Nurs. 1993;20(2):68-72.
12. Wynne R, Botti M, Stedman H, et al. Effect of three wound dressings on infection, healing comfort, and cost in patients with sternotomy wounds: a randomized trial. Chest. 2004;125(1):43-49.
13. Teshima H, Kawano H, Kashikie H, et al. A new hydrocolloid dressing prevents surgical site infection of median sternotomy wounds. Surg Today. 2009;39(10):848-854.
14. Morykwas MJ, Argenta LC, Shelton-Brown EI, et al. Vacuum-assisted closure: A new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38(6):553-562.
15. Morykwas MJ, Simpson J, Punger K, et al. Vacuum-assisted closure: State of basic research and physiologic foundation. Plast Reconstr Surg. 2006;117(7 SUPPL.).
16. Bakaeen FG, Haddad O, Ibrahim M, et al. Advances in managing the noninfected open chest after cardiac surgery: Negative-pressure wound therapy. J Thorac Cardiovasc Surg. 2019;157(5):1891-1903.e9.
17. Nherera LM, Trueman P, Schmoeckel M, Fatoye FA. Cost-effectiveness analysis of single use negative pressure wound therapy dressings (sNPWT) compared to standard of care in reducing surgical site complications (SSC) in patients undergoing coronary artery bypass grafting surgery. J Cardiothorac Surg. 2018;13(1).
18. Simek M, Chudoba A, Hajek R, et al. From open packing to negative wound pressure therapy. A critical overview of deep sternal wound infection treatment strategies after cardiac surgery. Biomed Pap. 2018;164(4).
19. Effect of the Negative Pressure Therapy Dressing Compared With Hydrogel Dressing. - ClinicalTrials.gov.
20. Singh DP, Gabriel A, Parvizi J, et al. Meta-analysis of comparative trials evaluating a single-use closed-incision Negative-Pressure Therapy System. Plast Reconstr Surg. 2019;143(1 Management of Surgical Incisions Utilizing ClosedIncision NegativePressure Therapy):41S-46S.
Received: April 4, 2022
Accepted: April 19, 2022
Published: April 25 , 2022.
To cite this article : Garrido-Martín P, Rodríguez-Fortúnez P, Montoto-López J, et al. Negative-Pressure Therapy versus Hydrocolloid Dressing on the Prevention of Surgical Wound Infections in Cardiac Surgery: A Randomised Controlled Trial Protocol. British Journal of Heart Diseases. 2022;4(1): 170 - 174. doi: 10.31488/bjhd.114
©2022 Garrido-Martín P, et al.
