Review Article / Open Access
DOI: 10.31488 /bjhd.110
Anticoagulation Therapy and Event Risk in Japanese Patients with Atrial Fibrillation: Insight from the J-RHYTHM Registry
Eitaro Kodani*1,6, Hirotsugu Atarashi2,6, Hiroshi Inoue3, Ken Okumura4, Takeshi Yamashita5, on behalf of the J- RHYTHM Registry Investigators.
1. Department of Internal Medicine and Cardiology, Nippon Medical School Tama-Nagayama Hospital,Tokyo,Japan.
2. Minami-Hachioji Hospital, Tokyo, Japan.
3. Saiseikai Toyama Hospital, Toyama, Japan.
4. Saiseikai Kumamoto Hospital, Kumamoto,Japan.
5. The Cardiovascular Institute, Tokyo, Japan.
6. Executive Office of the J-RHYTHM Registry and the J-RHYTHM Registry 2, Tokyo,Japan.
*Corresponding author: Eitaro Kodani, MD, PhD. Department of Internal Medicine and Cardiology, Nippon Medical School Tama-Nagayama Hospital.
Abstract
Since atrial fibrillation (AF) is a potent risk factor for thromboembolism, anticoagulation therapy is essential to prevent thromboembolism. Vitamin K antagonists (VKAs), mainly warfarin, have reportedly been able to reduce the risk of thromboembolism by 60%–70%. Although several non-VKA oral anticoagulants (NOACs) are currently available for the prevention of ischemic stroke and systemic embolism in patients with non-valvular AF (NVAF), VKAs remain to be used in patients with contraindication for NOACs such as severe renal impairment or valvular AF.The J-RHYTHM Registry was conducted as a nationwide prospective observational study to investigate the status of anticoagulation therapy with warfarin and the optimal anticoagulation therapy in Japanese patients with AF. A consecutive series of outpatients aged ≥20 years with AF of any type was recruited between January and July 2009, regardless of antithrombotic drug use. A total of 7,937 patients with AF were registered from 158 institutions. Patients were followed up for 2 years or until an event, such as thromboembolism, major hemorrhage, and all-cause death, whichever occurred first.The main analysis in 7,406 patients with NVAF showed the prothrombin time international normalized ratio (PT-INR) of 1.6–2.6 was safe and effective at preventing thromboembolic events in patients with NVAF, particularly patients aged ≥70 years. The PT-INR of 2.6–2.99 was also effective,but associated with a slightly increased risk of major hemorrhage. This AF registry has become a landmark study in Japan, and so far, more than 20 post hoc analyses have been published.In addition, the J-RHYTHM Registry 2 extended the follow-up period of the J-RHYTHM Registry for a total of 5 years, to investigate the effects of NOACs and the long-term effects of warfarin in Japanese patients with NVAF. This review overviews the J-RHYTHM Registry and the J-RHYTHM Registry 2.
Keywords: atrial fibrillation, anticoagulation, warfarin, noac, thromboembolism, major hemorrhage.
Introduction
Atrial fibrillation (AF) is likely to be developed in the population of advanced age. Therefore, the number of people with AF has been increasing worldwide along with the rise in aged population, and it is predicted to reach 5–16 million in the United States [1-3], and more than 1 million in Japan [4,5] in 2050. Since AF is a potent risk factor for thromboembolism [1,6], anticoagulation therapy is essential to preventing thromboembolism, specifically cardiogenic ischemic stroke that is likely to lead patients to poor prognosis. Vitamin K antagonists (VKAs), mainly warfarin, have reportedly been able to reduce the risk of thromboembolism by 60%–70% [7,8]. Although several non-VKA oral anticoagulants (NOACs, equal to direct oral anticoagulants [DOACs]) are currently available for the prevention of ischemic stroke and systemic embolism in patients with non-valvular AF (NVAF), VKAs remain to be used in patients with contraindication for NOACs such as severe renal impairment or valvular AF. Since anticoagulation therapy raises a risk of hemorrhagic complications [9], optimal anticoagulation therapy with well-balanced risk assessment for thromboembolic and hemorrhagic events should be chosen [10]. A therapeutic strategy, including anticoagulation therapy with a high-evidence level based on the results of randomized controlled trials (RCTs) or their meta-analysis is generally recommended. However, results from the RCTs are not always extrapolated to actual patient’s in general clinical setting, because patients with poor condition are usually excluded from RCTs. In contrast, results from registry studies are often helpful for physicians to determine the therapeutic strategy, although the evidence level is not high, because there are few exclusion criteria and diverse patients are included in the registry study from actual clinical setting [11]. Therefore, it is termed as real-world data.Several registry studies focusing real-world patients with AF have been initiated worldwide since the 2000s and some of these are currently ongoing. Among these, we had conducted the J-RHYTHM Registry [12], wherein more than 7,000 Japanese patients with AF were registered since January 2009 [13,14] and more than 20 post hoc analyses [15-38], have been published in addition to the main analysis [14] (Table 1).Thus, we herein overview the J-RHYTHM Registry, which has become a landmark registry study of AF in Japan, and the J-RHYTHM Registry 2 [39], which was the extended study of the J-RHYTHM Registry with an extended follow-up period for a total of 5 years, to investigate the effects of NOACs and the long-term effects of anticoagulation therapy with warfarin in Japanese patients with NVAF.
Table 1:Publications of the J-RHYTHM Registry and J-RHYTHM Registry 2
| J-RHYTHM Registry | First author | Year | Journal* | Short title [Reference] |
|---|---|---|---|---|
| Study design | Atarashi H. | 2011 | J Cardiol | J-RHYTHM Registry study design [12] |
| Baseline data | Atarashi H. | 2011 | Circ J | Status of anticoagulation in AF in Japan [13] |
| Baseline subanalysis | JRR invesrtigators | 2011 | Circ J | Warfarin use and INR control in AF patients [15] |
| Main analysis | Inoue H. | 2013 | Circ J | Target INR in NVAF [14] |
| Post hoc analyses | Inoue H. | 2014 | Am J Cardiol | Effect of gender on the prognosis of NVAF [16] |
| Okumura K. | 2014 | Circ J | CHA2DS2-VASc and HAS-BLED scores in Japanese [17] | |
| Inoue H. | 2014 | Circ J | Type of NVAF and thromboembolism [18] | |
| Yamashita T. | 2015 | J Cardiol | Warfarin anticoagulation intensity in Japanese NVAF [19] | |
| Kodani E. | 2015 | Circ J | Anticoagulation in valvular AF [20] | |
| Suzuki S. | 2015 | Circ J | Ischemic stroke in Japanese AF patients [21] | |
| Chishaki A. | 2015 | Thromb Res | Propensity score matching subanalysis [22] | |
| Ogawa H. | 2015 | Int J Cardiol | Antiplatelet therapy in Japanese patients with AF** [23] | |
| Tomita H. | 2015 | Circ J | Validation of CHA2DS2-VA in Japanese NVAF [24] | |
| Tomita H. | 2015 | Int J Cardiol | Risk factors for bleeding in Japanese NVAF** [25] | |
| Kodani E. | 2015 | Circ J | Non-valvular AF in elderly Japanese patients [26] | |
| Kodani E. | 2016 | J Stroke Cerebrovasc Dis | Secondary stroke prevention with warfarin in nonvalvular AF [27] | |
| Watanabe E. | 2016 | Int J Cardiol | Net clinical benefit of adding aspirin to warfarin [28] | |
| Inoue H. | 2016 | Am J Cardiol | BMI and prognosis of AF [29] | |
| Inoue H. | 2016 | Circ J | Regional differences in AF prognosis [30] | |
| Kodani E. | 2016 | J Am Heart Assoc | Blood pressure and events in nonvalvular AF [31] | |
| Kumagai N. | 2017 | Am J Cardiol. | Statin and thromboembolism in AF [32] | |
| Kodani E. | 2018 | Eur Heart J QCCO | Renal function and outcomes in non-valvular AF [33] | |
| Inoue H. | 2018 | Circ J | Renal dysfunction and warfarin control [34] | |
| Inoue H. | 2018 | Circ J | Time in therapeutic range and outcomes of NVAF [35] | |
| Kodani E. | 2019 | Circ J | Digitalis and mortality in NVAF [36] | |
| Kodani E. | 2020 | Int J Cardiol | Hemoglobin and outcomes of non-valvular AF [37] | |
| Kodani E. | 2020 | Int J Cardiol Heart Vasc | CrCl versus eGFR for outcomes in non-valvular AF [38] | |
| J-RHYTHM Registry 2 | Kodani E. | 2016 | Circ J | Beneficial effect of NOACs in NVAF [39] |
* Abbreviated to the style used in Index Medicus (http://www.nlm.nih.gov/tsd/serials/lji.html). ** Letter to the Editor JRR, J-RHYTHM Registry; QCCO, Qual Care Clin Outcomes; AF, atrial fibrillation; INR, international normalized ratio; NVAF, non-valvular atrial fibrillation; BMI, body mass index; CrCl, creatinine clearance; eGFR, estimated glomerular filtration rate; NOACs, non-vitamin K antagonist oral anticoagulants.
Differences between randomized controlled trial and registry study
It is necessary to understand the differences between RCT and observational study to interpret the results of registry studies properly. In RCTs, patients are assigned randomly into the therapeutic and control groups, and the prespecified endpoints such as thromboembolism, major hemorrhage, and all-cause death are investigated prospectively for the defined observation period. Since patient characteristics become comparable owing to randomization, a simple comparison using unadjusted data can be allowed to determine the differences in event rates and drug effects among groups. Eligible subjects in RCTs, specifically in phase III clinical trials prior to the approval of novel drugs, are often far from those in real-world clinical setting since the trials are achieved among limited patients who met the strict enrollment criteria and were under the strict control. In contrast, in registry study, subjects are generally enrolled consecutively without exclusion criteria and do not receive any intentional intervention. Medications, including antithrombotic drugs, are determined by participating physicians and are sometimes out of their approved doses or indications. Since a selection bias always exists in an observational study, patient characteristics in the therapeutic group are not similar to those in the control group. When comparing outcome events between the groups with different patient characteristics, it is necessary to adjust for confounding factors using some statistical methods such as multivariate Cox proportional hazard model and propensity score matching. Therefore, the results in the adjusted model often differ from those in the unadjusted model. Note that it is difficult to adjust completely for unmeasured variables and unrecognized risk factors even after adjusting maximally for known confounding factors such as components of the conventional risk scores. Therefore, RCT is able to prove hypothesis, whereas observational study is just hypothesis-generating in nature. That is why evidence level of registry study is lower than that of RCT. In addition, since there are various subjects and participating physicians in registry studies, it seems common and rather inevitable that different registry studies with different populations result in different results.
Study design of the J-RHYTHM registry
The J-RHYTHM Registry was conducted as a nationwide prospective observational study to investigate the status of anticoagulation therapy and the optimal anticoagulation therapy in Japanese patients with AF [12]. The study design and baseline patient characteristics have been reported elsewhere [12,13]. Briefly, the study protocol conformed to the Declaration of Helsinki and was approved by the ethics committee of each participating institution. Written informed consent was obtained from all participants at the time of enrollment. A consecutive series of outpatients aged ≥20 years with AF of any type was recruited since January 2009, regardless of the antithrombotic drug use. Only patients who have maintained a sinus rhythm for more than 1 year were excluded. All drugs and their dosages were selected at the discretion of the treating physicians. Patients with valvular AF (mechanical heart valve and mitral stenosis) were analyzed separately [20] from those with NVAF [14]. Risk factors for thromboembolism were evaluated using the CHADS2 score (congestive heart failure, hypertension, age ≥75 years, and diabetes mellitus for 1 point and history of stroke or transient ischemic attack [TIA] for 2 points) [40]. Anticoagulation intensity was determined at the time of enrollment (baseline) and at each follow-up visit using the prothrombin time international normalized ratio (PT-INR) in patients receiving warfarin (Table 2). Time in therapeutic range (TTR) was also calculated using the Roosendaal method [41] to evaluate the overall quality of anticoagulation therapy during the follow-up period. Note that the target PT-INR levels were set at 1.6–2.6 for patients aged ≥70 years and at 2.0–3.0 for those aged <70 years according to the Japanese guidelines [42].Patients were followed up for 2 years or until an endpoint event, whichever occurred first. The primary endpoints were defined as thromboembolism, including symptomatic ischemic stroke, TIA, and systemic embolism; major hemorrhage, including intracranial hemorrhage, gastrointestinal hemorrhage, and other hemorrhages requiring hospitalization; and all-cause death. In some post hoc analyses, cardiovascular death [18,20,22,26,27,29,32,33,36,39] and the composite of thromboembolism, major hemorrhage, and all-cause death [33-35,37,38], whichever occurred first for each patient, were also evaluated. The diagnostic criteria for each event have been described elsewhere [12,13]. If any event occurred during the follow-up period, PT-INR and blood pressure (BP) at the time prior to and closest to the event were recorded [12,14,31].
Table 2:
| Evaluations at baseline | Evaluations during 2-year follow-up |
|---|---|
| Demographic information (age, sex) | |
| Medical history | |
| Risk factors for thromboembolism (CHADS2 score) | |
| Physical examination | Physical examination |
| Peripheral blood pressure | Peripheral blood pressure |
| Heart rate | Heart rate |
| Laboratory information | Laboratory information |
| International normalized ratio | International normalized ratio |
| Platelet count | |
| Serum creatinine | |
| Hemoglobin | |
| Medications | Medications |
| Warfarin | Warfarin |
| Antiplatelets | Antiplatelets |
| Antihypertensives | |
| Medication for diabetes mellitus | |
| Antiarrhythmics |
(Adapted from Atarashi et al., J Cardiol 2011; 57: 95-9 [12].)
Differences between the J-RHYTHM registry and the Fushimi AF registry
The Fushimi AF Registry [43,44] is another major registry study in Japanese patients with AF, which was initiated in 2011 just before the approval of the first NOAC dabigatran, and is often compared with the J-RHYTHM Registry.
In contrast to the J-RHYTHM Registry, the Fushimi AF Registry was conducted by predominantly general practitioners, and patients with AF, visiting general hospital or clinic, were widely recruited from localized area in Fushimi, Kyoto, Japan. Therefore, risk of thromboembolism in the Fushimi AF Registry was higher than that in the J-RHYTHM Registry; that is, the mean CHADS2 scores were 2.1±1.4 and 1.7±1.2, respectively [12,43]. In addition, PT-INR values were assessed only at the time of enrollment in the Fushimi AF Registry [43]. In this review, some results of the Fushimi AF Registry will be compared with those in the J-RHYTHM Registry, as appropriate.
Patients Characteristics at Baseline
Table 3:Baseline characteristics of the J-RHYTHM Registry
| Number of patients | 7,937 |
| Age, years | 69.7 ± 9.9 |
| Sex, men | 68.9% |
| Type of atrial fibrillation | |
| Paroxysmal | 37.1% |
| Persistent | 14.4% |
| Permanent | 48.5% |
| Comorbidities | |
| Coronary artery disease | 10.1% |
| Valvular disease | 13.7% |
| Mitral stenosis/Prosthetic valve | 3.5%/3.1% |
| Cardiomyopathy | 8.3% |
| HCM/DCM | 3.4%/4.9% |
| Congenital heart disease | 1.3% |
| COPD | 1.7% |
| Hyperthyroidism | 1.7% |
| CHADS2 score | 1.7 ± 1.2 |
| 0 | 15.6% |
| 1 | 34.0% |
| ≥ 2 | 50.4% |
| Risk factors for stroke | |
| C: Heart failure | 30.0% |
| H: Hypertension | 59.1% |
| A: Age ≥ 75 years | 32.8% |
| D: Diabetes mellitus | 18.2% |
| S2: Stroke/TIA | 14.0% |
| Heart rate, /min | 72.5 ± 13.2 |
| Systolic blood pressure, mmHg | 125.7 ± 16.1 |
| Diastolic blood pressure, mmHg | 73.1 ± 11.1 |
| Antithrombotic therapy | |
| Warfarin | 87.3% |
| PT-INR | 1.92 ± 0.50 |
| Achievement rate of target PT-INR* | 53.1% |
| < 70 years (rate of PT-INR 2.0–3.0) | 37.0% |
| ≥ 70 years (rate of PT-INR 1.6–2.6) | 66.2% |
| Antiplatelet | 25.9% |
| Aspirin | 22.3% |
| Warfarin+ aspirin | 15.7% |
Data expressed as percentage or mean ± standard deviation. HCM, hypertrophic cardiomyopathy; DCM, dilated cardiomyopathy; COPD, chronic obstructive pulmonary disease; TIA, transient ischemic attack; PT-INR, prothrombin time international normalized ratio. * Target INR was 2.0–3.0 (< 70 years) or 1.6–2.6 (≥ 70 years). (Adapted from Atarashi et al. Circ J. 2011; 75: 1328-33 [13].)
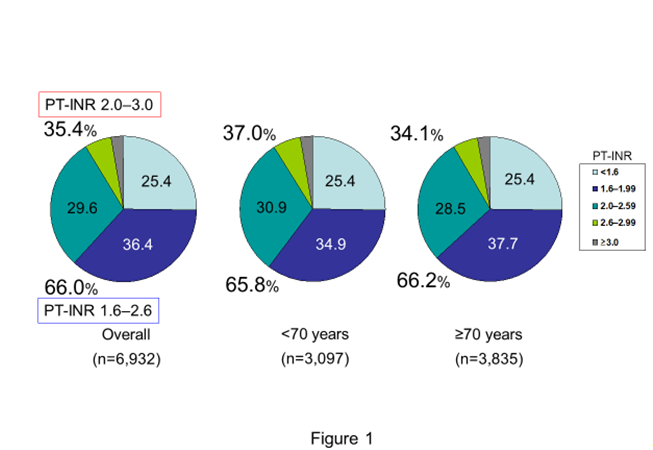
Figure 1:Distribution of baseline PT-INR in the J-RHYTHM Registry PT-INT, prothrombin time international normalized ratio Generated from Atarashi et al., Circ J. 2011; 75: 1328-33 [13].

Figure 2:Patient disposition of the J-RHYTHM Registry AF, atrial fibrillation
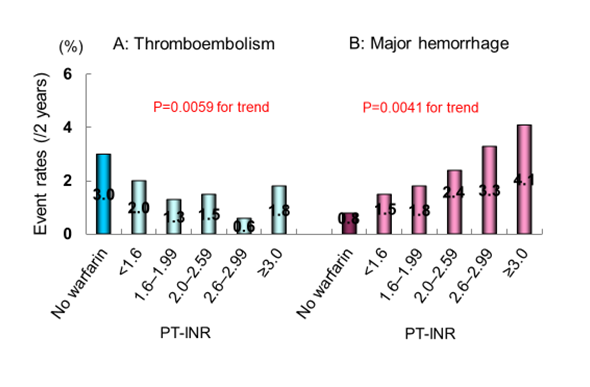
Figure 3:Two-year event rates of thromboembolism (A) and major hemorrhage (B). PT-INT, prothrombin time international normalized ratio. Generated from Inoue et al., Circ J. 2013; 77: 2264-70 [14].
A total of 7,937 patients with AF were registered from 158 institutions until July 2009. Baseline characteristics in the J-RHYTHM Registry are shown in Table 3. A notable feature of this registry was that anticoagulation therapy with warfarin was performed more frequently (87.3%) compared with that in the Fushimi AF Registry (50.5%) [43,44] because most of the participating physicians specialized in cardiology and in the management of cardiac arrhythmias in the J-RHYTHM Registry. On the contrary, antiplatelets were less prescribed in the J-RHYTHM Registry than in the Fushimi AF Registry (25.9% vs. 31.3%) [12,43]. The most impressive result of the baseline data analyses was that the distribution of baseline PT-INR values was extremely similar between patients aged <70 years and those aged ≥70 years [13] (Figure 1). This suggested that most Japanese physicians managed patients with AF receiving warfarin aiming the PT-INR levels around 2.0 even in younger patients aged <70 years. The factors for the hesitation of warfarin use included age <60 years, paroxysmal AF, and antiplatelet use [15]. Diabetes mellitus and antiplatelet use were negative factors to achieve target PT-INR in the J-RHYTHM Registry [15].
Event analyses
Of the 7,937 entire patients with AF, a total of 7,406 patients with NVAF were analyzed after excluding those with valvular AF and who lost to follow-up [14] (Figure 2). Incidence rates of clinically important events were relatively low on the whole, that is, 0.8% per year for thromboembolism and 1.1% per year for major hemorrhage [14,17], compared with 2.7% and 1.5%, respectively, in the Fushimi AF Registry [44]. The incidence of thromboembolism and all-cause death was significantly lower and that of major hemorrhage was higher in patients receiving warfarin than in those not receiving anticoagulation therapy [14,17,22]. When patient backgrounds were equalized using propensity score matching, in other words, when mimicking RCT, the incidence of major hemorrhage in patients receiving warfarin was comparable, whereas that of thromboembolism and all-cause death was significantly lower in patients receiving warfarin, compared with those not receiving warfarin [22]. These findings suggested that anticoagulation therapy with warfarin might have beneficial effects to reduce the incidence of thromboembolism and all-cause death and might not induce hemorrhagic complications, although the J-RHYTHM Registry was not an RCT. In contrast, anticoagulation therapy, mainly warfarin, did not affect the incidence of either thromboembolism or major hemorrhage in the Fushimi AF Registry [44]. It was probably due to the lower rate of anticoagulation therapy in that registry [44]. Regarding baseline PT-INR, thromboembolism occurred less frequently in patients receiving warfarin with the baseline PT-INR of 1.6–2.99 than in those not receiving anticoagulation therapy, whereas major hemorrhage occurred more frequently in the PT-INR ≥2.6 [14] (Figure 3). Thus, the PT-INR of 1.6–2.6 was safe and effective at preventing thromboembolic events in patients with NVAF, particularly in those aged ≥70 years. The PT-INR of 2.6–2.99 was also effective, but associated with a slightly increased risk of major hemorrhage [14]. The estimated association between PT-INR and event risk for ischemic stroke/systemic embolism was similar to that in the Western countries, whereas that for intracranial hemorrhage (ICH) shifted leftward (lower) by a PT-INR of approximately 0.5 in Japanese patients with NVAF [19], indicating that the optimal therapeutic range of PT-INR would be narrower in Japanese patients with NVAF treated with warfarin than those in Caucasians. Accordingly, the PT-INR of 1.6–2.6, which is a current target for patients aged ≥70 years in Japan [42], might be optimal for all Japanese patients with NVAF. However, TTR should be kept more than 60% to obtain the satisfactory effect of warfarin in preventing thromboembolism and all-cause death [35]. Several previous studies have also demonstrated that the TTR needs to be maintained above 60%–75% for a significant reduction in stroke and systemic embolism [45-47]. In addition, major hemorrhage was observed more frequently in patients with low TTR of <40% (hazard ratio [HR] 5.57, 95% confidence interval [CI] 2.04–15.25, compared with no-warfarin) in the J-RHYTHM Registry [35]. Some confounding factors not determined in the present analysis could have been involved in the association between TTR <40% and major hemorrhagic events.
Post hoc Analyses on Risk Assessment
Since the CHA2DS2-VASc score [48] was adopted as a risk assessment score for thromboembolism in the 2012 focused update of the European Society of Cardiology (ESC) Guidelines for the Management of Atrial Fibrillation [49], this risk score has been used worldwide and also adopted in the 2016 ESC Guidelines [50] and in the other guidelines [51-54]. It comprises age ≥75 years and prior stroke, TIA, or thromboembolism for 2 points and other components of the CHADS2 score, vascular disease (prior myocardial infarction, peripheral artery disease, or aortic plaque), age 65–74 years, and sex category (female sex) for 1 point. Certainly, these components were identified as significant risk factors for ischemic stroke, TIA, and systemic embolism in Caucasians [48,55]. In contrast, the CHADS2 score [40] has been adopted in the Japanese guidelines [42] to simplify the risk assessment for thromboembolism in patients with NVAF. The HAS-BLED score (hypertension [systolic BP >160 mmHg], abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly [>65 years], drugs/alcohol concomitantly) [9] has also been widely used for the assessment of bleeding risks during anticoagulation therapy. Since risk factors for adverse events in patients with AF might be specific in race, ethnicity, and generation, these risk scores should be validated for the suitability in current Japanese patients with NVAF. Therefore, several post hoc analyses were performed to validate conventional risk scores and to identify other risk factors for adverse events using the data of the J-RHYTHM Registry.
Validation of conventional risk scores
In a sub analysis of the J-RHYTHM Registry on the validation of the CHA2DS2-VASc and the HAS-BLED scores in Japanese patients with NVAF [17], the efficacy of warfarin treatment in reducing thromboembolic events was observed in patients with the CHADS2 score of 1 (odds ratio [OR] 0.39, 95% CI 0.18–0.81) and ≥2 (OR 0.43, 95% CI 0.25–0.73) and with the CHA2DS2-VASc score of 1 (OR 0.25, 95% CI 0.06–1.01) and ≥2 (OR 0.45, 95% CI 0.29–0.70). There was no significant difference in the rate of thromboembolism in patents with the CHA2DS2-VASc score of 0; thus, the CHA2DS2-VASc score was useful for identifying patients at truly low risk of thromboembolism. The HAS-BLED score ≥3 was also useful for identifying patients at high risk of major bleeding [17]. Thus, these risk scores were useful for Japanese patients with NVAF as well as for Caucasians. However, the predictive ability of the CHADS2 score for thromboembolism assessed by C-statistics was superior to that of the CHA2DS2-VASc score (0.638 vs. 0.595, P=0.043) in another sub analysis of the J-RHYTHM Registry [24], indicating that the CHADS2 score rather than the CHA2DS2-VASc score may be more suitable for Japanese patients with NVAF. Indeed, in a pooled analysis of the 3 major Japanese AF registries (J-RHYTHM Registry, Fushimi AF Registry, and Shinken Database) [21], the 3 components in the CHA2DS2-VASc score (vascular disease, age 65–74 years, and female sex) added to the CHADS2 score were not associated with ischemic stroke in Japanese patients with NVAF not receiving anticoagulation therapy.
Thus, the current recommendation in Japanese guidelines, in which the CHADS2 score is adopted [42], may be appropriate for Japanese patients with NVAF, despite the CHA2DS2-VASc score is recommended in other countries [49,50,52-54].
Evaluation of each component of the chads2 score
Congestive heart failure
Congestive heart failure is defined as recent exacerbated heart failure within the past 100 days [40,56]. However, it is generally determined by physicians based on symptoms, examinations, and medications for heat failure because the definition of exacerbation is vague in a clinical setting. In the J-RHYTHM Registry, 30% of patients were diagnosed with heart failure [13] (Table 3). Although an analysis focusing specifically on heart failure has not been achieved yet, heart failure was used as an adjusting variable in several post hoc analyses. Consequently, heart failure was not identified as an independent risk factor for thromboembolism in patients with NVAF, including those receiving warfarin [18,29-36] or in those not receiving anticoagulation therapy [21,23]. In contrast, heart failure was consistently an independent risk factor for all-cause death [18,29,33-37] and cardiovascular death [18,29,33-37] in Japanese patients with NVAF. The reasons why heart failure was not identified as a significant risk factor for thromboembolism include the following: the current standard therapeutic drugs for heart failure differ from those in the 1990s, heart failure is a stronger risk factor for all-cause and cardiovascular deaths than thromboembolism [18,29,33-37,57], and the severity or duration of heart failure was not considered. Certainly, in a subanalysis of the ENGAGE AF-TIMI 48 (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48) trial [58], severe heart failure with New York Heart Association class III or IV was a significant risk factor for thromboembolism (HR 1.45, 95% CI 1.12–1.88). In addition, the incidence of stroke or systemic embolism markedly increased in the 30 days after admission for heart failure (HR 12.0, 95% CI 4.59–31.98) in a subanalysis of the Fushimi AF Registry [59].
Hypertension
Hypertension is a risk factor for stroke even in patients with its history and/or adequate BP control under treatment in the Framingham study [60]. In subanalyses on hypertension of the phase III trials using NOACs, hypertension was a significant risk factor for stroke or systemic embolism in the ROCKET-AF (Rivaroxaban Once-daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) trial [61] and the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial [62], but not in the RE-LY (Randomized Evaluation of Long Term Anticoagulant TherapY) trial [63]. In a subanalysis of the J-RHYTHM Registry on hypertension and BP values [31], hypertension (including its history and/or under treatment) was an independent risk factor for major hemorrhage (HR 1.52, 95% CI 1.05–2.21) but not for thromboembolism. Systolic and diastolic BP values at the time of enrollment were well-controlled on average (Table 3) [13,31]. Thus, either systolic or diastolic BP value at the time of enrollment was not an independent risk factor for thromboembolism or major hemorrhage. In contrast, the systolic BP ≥136 mmHg at the time closest to the event was a significant risk factor for both events compared with systolic BP <114 mmHg as a reference (OR 2.88, 95% CI 1.75–4.74 for thromboembolism and OR 1.61, 95% CI 1.02–2.53 for major hemorrhage) [31] (Figure 4). A subanalysis of the Fushimi AF Registry on hypertension [64] also showed that event rates in patients with hypertension were comparable to those without it, but the incidence rates of stroke or systemic embolism and hemorrhagic stroke in patients with a baseline systolic BP ≥150 mmHg were significantly higher than those with adequate BP control [64].Since patients with higher BP had consistently indicated higher event rates in all studies [31,61-64], appropriate BP control is definitely important and may result in reduced risks of both thromboembolism and major hemorrhage in patients with NVAF. The association between the visit-to-visit BP variability during the follow-up period and adverse events in patients with NVAF was reported in a post hoc analysis of the AFFIRM (Atrial Fibrillation Follow-Up Investigation of Rhythm Management) Study [65] that the third and highest quartiles of systolic BP-standard deviation were independently associated with a higher risk for stroke (HR 1.85 and HR 2.33, respectively). This issue could be a next task to be clarified in the J-RHYTHM Registry.
Age ≥75 Years
Age ≥75 years is indicated as a strong risk factor for thromboembolism among the components of the CHADS2 score [40,55,66]. In a subanalysis of the J-RHYTHM Registry on older patients and PT-INR [26], age ≥75 years was also a strong risk factor for thromboembolism. In that study, composite events of thromboembolism and major hemorrhage were less frequent in patients with the PT-INR of 1.6–2.6, even in those aged 75–84 years and also ≥85 years [26], indicating that anticoagulation therapy with warfarin could have beneficial effects even in very old patients with NVAF if PT-INR values were kept between 1.6 and 2.6.

Figure 4:Odds ratios for thromboembolism (A) and major hemorrhage (B) in each quartile of systolic BP Multivariate logistic regression model using systolic BP quartiles at the time closest to the event per at the end of the follow-up period. Odds ratios were adjusted for the components (except hypertension) of the CHA2DS2-VASc score and use of warfarin and antiplatelet. BP, blood pressure at the time prior to and closest to an event or at the end of follow-up Generated from Kodani et al., J Am Heart Assoc. 2016; 5: e004075 [31].
Diabetes Mellitus
Diabetes mellitus was identified as a risk factor for thromboembolism in some studies in Caucasians [7,55]. However, in the pooled analysis of the 3 major Japanese AF registries (J-RHYTHM Registry, Fushimi AF Registry, and Shinken Database) [21], diabetes mellitus was not identified as a risk factor for ischemic stroke in Japanese patients with NVAF not receiving anticoagulation therapy. Therefore, it remains controversial whether diabetes mellitus is certainly an independent risk factor for thromboembolism. Possible reasons for the discrepant results include differences in duration of diabetes mellitus, blood glucose levels, and oral hypoglycemic agents among the studies.
In a subanalysis of the J-RHYTHM Registry on the effect of stain use in patients with NVAF and diabetes mellitus [32], the incidence of thromboembolism in those receiving statin in addition to warfarin was lower than that in those receiving warfarin only [32]. Accordingly, the concomitant use of warfarin and statin might have clinically beneficial effects for thromboembolism in patients with NAVF and diabetes mellitus.
Prior stroke or transient ischemic attack
Prior stroke or TIA is recognized as a stronger risk for thromboembolism than other risk factors [27,55,67,68]; thus, it has been assigned for 2 points in both the CHADS2 [40] and the CHA2DS2-VASc scores [48]. In a subanalysis of the J-RHYTHM Registry on the secondary prevention of stroke [27], prior stroke or TIA was consistently a stronger risk factor for thromboembolism. In that study, composite events of thromboembolism and major hemorrhage were less frequent in patients with the PT-INR of 1.6–2.6, even in those with prior stroke or TIA [27], indicating that anticoagulation therapy with warfarin with PT-INR values between 1.6 and 2.6 could be beneficial for the secondary prevention of stroke.
Evaluation of Factors Not Included in the CHADS2 Score
As shown before, either vascular disease, age 65–74 years, or female sex in the CHA2DS2-VASc score was not a significant risk for ischemic stroke in Japanese patients with NVAF not receiving anticoagulation therapy in the pooled analysis of the 3 major Japanese AF registries including the J-RHYTHM Registry [21]. In post hoc analyses of the J-RHYTHM Registry, several factors not included in the CHADS2 score such as female sex, AF type, body mass index (BMI), renal Impairment, hemoglobin level and platelet count, antiplatelet use, digitalis use, and geographical region, were also evaluated [16,23,28-30,33,36,37,69].
Sex category (female sex)
Female sex was identified as a risk factor for thromboembolism in the Framingham study [7] and the Swedish Cohort Atrial Fibrillation stud [55]. However, in other studies [66,70,71], it was not identified as a solo risk factor in AF patients aged <65 years without other organic diseases, to whom anticoagulant therapy would not be needed. In a subanalysis of the J-RHYTHM Registry on sex differences in adverse events [16], female sex was not identified as a risk factor in Japanese patients with NVAF, a finding consistent with other studies in Caucasians [66,70,71]. In a validation study of the J-RHYTHM Registry on the CHA2DS2-VA score (excluding female sex from the CHA2DS2-VASc score) [24], the predictive ability of the CHA2DS2-VA score for thromboembolism assessed by C-statistics was superior to that of the CHA2DS2-VASc score (0.624 vs. 0.595, P=0.029). Thus, being female should be considered as not a risk, but a modifier of other risk factors independent of sex [24,72].
Atrial fibrillation typ
Based on the results of the ACTIVE W (Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events) Substudy [73], AF type had been thought no association with the incidence of ischemic stroke. In a subanalysis of the J-RHYTHM Registry on AF type [69], the results supported the ACTIVE W Substudy; that is, the risk for thromboembolism in patients with permanent AF was comparable to those with paroxysmal AF after adjusting multiple confounding factors [69]. However, several subsequent post hoc analyses using AF type as a covariate on multivariate analysis revealed that the risk of thromboembolism in patients with paroxysmal AF was significantly lower than those with permanent AF even in the same study subjects [29,30,35]. Thus, the results of multivariate analysis should be interpreted cautiously, since they often differ among studies depend upon the differences in selected explanatory variables for the adjustment as in this case. In a subanalysis of the Fushimi AF Registry [74], the risk for thromboembolism in patients with persistent or permanent AF was higher than those with paroxysmal AF. In addition, in the J-RISK AF study, a pooled analysis of the 5 major Japanese AF registries, including J-RHYTHM Registry, Fushimi AF Registry, Shinken Database, Keio Interhospital Cardiovascular Studies, and Hokuriku-Plus AF Registry (N=12,289), also demonstrated that the risk ratio of persistent or permanent AF for ischemic stroke was significantly high (HR 1.59, 95% CI 1.21–2.10) compared with paroxysmal AF (Table 4) [75]. Differences in duration and frequency of paroxysmal AF episodes might also have contributed to the discrepant results.
Table 4:Risk factors for ischemic stroke in Japanese patients with AF
| Characteristic | HR (95% CI) | P value |
|---|---|---|
| Age | ||
| < 75 years | 1 [Reference] | |
| 75–84 years | 1.74 (1.32-2.30) | < 0.001 |
| ≥ 85 years | 2.41 (1.63-3.56) | <0.001 |
| Hypertension | 1.60 (1.15-2.23) | 0.006 |
| Previous stroke | 2.75 (2.09-3.62) | <0.001 |
| Persistent or permanent atrial fibrillation | 1.59 (1.21-2.10) | 0.001 |
| Body mass index < 18.5 kg/m2 | 1.55 (1.05-2.29) | 0.03 |
| No oral anticoagulant | 1.86 (1.40-2.47) | <0.001 |
Stepwise Cox proportional hazard model Pooled analysis of 5 major Japanese atrial fibrillation registries including J-RHYTHM Registry, Fushimi AF Registry, Shinken Database, Keio Interhospital Cardiovascular Studies, and Hokuriku-Plus AF Registry (N=12,289). HR, hazard ratio; CI, confidence interval (Adapted from Okumura et al. JAMA Netw Open 2020; 3: e202881 [75].)
Body Weight or Body Mass Index
Although the association between body weight (BW) and adverse events in patients with AF have been reported [76-79], results were inconsistent. In a study from China, the incidence rate of thromboembolism was higher in AF patients with obese than in those with underweight or normal weight [77]. In contrast, obesity was not associated with poor outcomes among subjects with established AF, but rather associated with lower mortality [76,77,80]. This phenomenon is known as the obesity paradox [81,82]. Furthermore, several studies did not find a significant association between BW and embolic risk [76,78,79]. Even in Japanese patients with AF, results were inconsistent [29,83]. In a subanalysis of the Fushimi AF Registry [83], a low BW of ≤50 kg was identified as an significant risk factor for ischemic stroke/systemic embolism in Japanese patients with AF. In contrast, in a subanalysis of the J-RHYTHM Registry on BMI [29], low BMI of <18.5 kg/m2 was identified as a stronger risk factor (compared with normal BMI of 18.5–24.9 kg/m2) for all-cause death (HR 2.40, 95% CI 1.59–3.63), rather than for thromboembolism (HR 1.22, 95% CI 0.63–2.38). The possible reason is thought that patients with low BW or low BMI are often complicated with sarcopenia, frailty, and renal impairment in patients with AF, specifically in elderly patients. Interestingly, the obesity paradox for mortality was observed in this study, demonstrating that mortality in patients with slightly high BMI of 25–29.9 kg/m2 was lower than those with normal BMI of 18.5–24.9 kg/m2 [29], as in previous reports [76,77,80].
Renal impairment
Renal impairment is also reportedly a risk factor for stroke or all-cause death in patients with AF [84,85] as well as in the general population [86,87]. It is also true in Japanese patients with AF [33,88]. In a subanalysis of the Fushimi AF Registry on renal function, incidence of stroke/systemic embolism and major bleeding in patients with low creatinine clearance (CrCl) of <30 mL/min was significantly higher compared with those with CrCl ≥50 mL/min [88]. In contrast, in a subanalysis of the J-RHYTHM Registry on renal function [33], low CrCl of <30 mL/min was a stronger risk factor (compared with CrCl ≥80 mL/min) for all-cause death (HR 6.44, 95% CI 3.03–13.7), rather than for thromboembolism (HR 1.69, 95% CI 0.62–4.62). The reason may be similar to BMI since BMI and renal impairment often coexist. Low BMI (<18.5 kg/m2) and severe renal impairment (CrCl <30 mL/min) could be a competing risk against all-cause death. Cumulative rates of composite event including thromboembolism, major hemorrhage and all-cause death showed evident trends significantly across the 4 CrCl groups [33] (Figure 5).In addition, the association between CrCl and TTR was found in patients with NVAF aged ≥70years [34]. Low CrCl of <30 mL/min was independently associated with low TTR of <65%. For thromboembolism, low TTR of <65% was a stronger risk (HR 2.26, 95% CI 1.37–3.72 compared with TTR ≥65%) than low CrCl of <30 mL/min (HR 1.68, 95% CI 0.41–1.85 compared with CrCl of ≥80 mL/min), whereas both low TTR and low CrCl were independently associated with an increased risk of all-cause death (low TTR, HR 1.60, 95% CI 1.07–2.38; low CrCl, HR 5.32, 95% CI 1.56–18.18) and composite events (low TTR, HR 1.58, 95% CI 1.22–2.04; low CrCl, HR 2.03, 95% CI 1.10–3.76) [34]. In general, CrCl is used for dose-adjustments of NOACs in patients with NVAF, and estimated glomerular filtration rate (eGFR) is adopted for the diagnosis of chronic kidney disease [89]. Since the predictive ability of CrCl versus eGFR for the outcomes in patients with NVAF remains controversial, the C-statistics of CrCl were compared with those of eGFR in a subanalysis of the J-RHYTHM Registry [38]. The C-statistics of CrCl for thromboembolism, major hemorrhage, and all-cause death were 0.609, 0.599, and 0.746; and those of eGFR were 0.542, 0.573, and 0.677, respectively. Thus, CrCl was superior to eGFR in the prediction of adverse outcomes in patients with NVAF, specifically of thromboembolism and all-cause death [38].
Hemoglobin level and platelet count
Hemoglobin concentration or anemia is reportedly associated with poorer outcomes and higher mortality in patients with various cardiac diseases such as coronary artery diseases after percutaneous coronary intervention [90,91], chronic heart failure [92], and AF [93,94]. A lower platelet count is also reportedly associated with a lower risk of stroke but a higher risk of bleeding events [95]. In a subanalysis of the J-RHYTHM Registry on hemoglobin level and platelet count [37], a lower hemoglobin level (< 12.0 g/dL) was an independent risk factor for all-cause death (HR 2.19, 95% CI 1.52–3.17) and composite events (HR 1.61, 95% CI 1.25–2.07), but not for thromboembolism or major hemorrhage in Japanese patients with NVAF. In contrast, platelet count was not independently associated with any outcome event after adjusting the confounding factors [37]. A recent subanalysis of the Fushimi AF Registry on hemoglobin level [96] also demonstrated that a lower hemoglobin level .

Figure 5:The Kaplan-Meier curves for the composite of thromboembolism, major hemorrhage, and all-cause death in the 4 CrCl groups. CrCl, creatinine clearance (mL/min) Cited from Kodani et al., Eur Heart J Qual Care Clin Outcomes. 2018; 4: 59-68 [33].
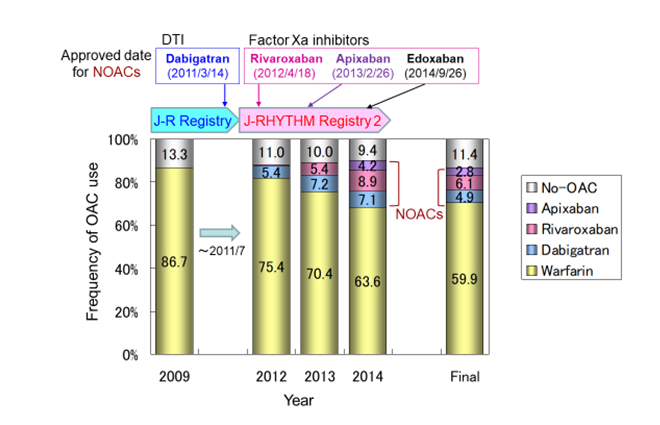
Figure 6:Changes in anticoagulation therapy during the extended follow-up period NOAC, non-vitamin K antagonist oral anticoagulant; DTI, direct throm- bin inhibitor; OAC, oral anticoagulant; J-R, J-RHYTHM. Generated from Ko- dani et al., Circ J. 2016; 80: 843-51 [39].
Antiplatelet use
Aspirin was shown to be inferior to warfarin or apixaban in preventing stroke in Caucasian patients with AF [97,98]. Since it was also shown in Japanese patients with NVAF that a low-dose aspirin was comparable to placebo in preventing stroke and rather caused a marginally increased risk of major bleeding in the JSAT (Japan Atrial Fibrillation Stroke Trial) Study [99], an antiplatelet use has not been recommended for the prevention of stroke in patients with NVAF in Japanese guidelines [42]. However, an antiplatelet was prescribed to many patients with AF in actual clinical setting in Japan [13,43] and China [100]. In a pooled analysis of the 3 major Japanese AF registries (J-RHYTHM Registry, Fushimi AF Registry, and Shinken Database) to assess the benefit and the risk of antiplatelet therapy in Japanese patients with AF who were not receiving anticoagulation therapy [23], ischemic stroke was more frequent in those receiving antiplatelet than those not receiving it. However, antiplatelet use was not independently associated with the incidence of thromboembolism or major hemorrhage after adjusting multiple confounding factors [23]. In contrast, among patients receiving anticoagulation therapy with warfarin, the incidence of thromboembolism was comparable and that of major hemorrhage was higher in those receiving both aspirin and warfarin compared with those receiving warfarin only. Positive net clinical benefit of adding aspirin to warfarin was obtained in only high-risk patients with the CHA2DS2-VASc score ≥2 and the HAS-BLED score ≥3 in the J-RHYTHM Registry [28].
Digitalis use
Digitalis, mainly digoxin, which has been used for ventricular rate control and heart failure management in patients with AF, is reportedly a poor prognostic factor [101]. In a subanalysis of the J-RHYTHM Registry on digitalis use and mortality [36], digitalis use was associated with all-cause death in the crude model, whereas it was not proven in the propensity score matching model. Several clinical confounding factors might contribute to increased mortality in patients with NVAF treated with digitalis. Patient condition, comorbidities, and digoxin concentration were more strongly associated with patient prognosis, rather than digoxin use itself [36,102].
Geographical region
Several efforts were made to avoid the uneven regional distribution of the study population, which were prespecified that patient registration was performed in proportion to the population density from each of the 10 Japanese geographical regions, and a maximum of 100 patients were allowed to enroll in each institution [12]. Nevertheless, there were differences in patient characteristics, frequency of anticoagulation therapy, PT-INR, and incidence rate of thromboembolism among 10 geographical regions, and geographical region itself was independently associated with the incidence of thromboembolism [30].
Hemorrhagic risk
According to the results from a sub analysis of the RE-LY trial [103], older age (≥75 years), low BW (≤50 kg), renal impairment (CrCl ≤50 mL/min), and antiplatelet use have been identified as major risk factors for hemorrhagic events during anticoagulation therapy. In a post marketing surveillance (PMS) study, the J-Dabigatran Surveillance, in Japanese patients with NVAF receiving dabigatran, age, prior stroke/TIA/systemic embolism, myocardial infarction, gastrointestinal bleeding, and p-glycoprotein inhibitor use were identified as independent risk factors for major bleeding [104]. In a subanalysis of the J-RHYTHM Registry on the risk factors for bleeding events during anticoagulation therapy with warfarin [25], the highest risk factor for major hemorrhage among the components of the HAS-BLED score [9], was prior bleeding (OR 5.03, 95% CI 3.13–7.82) followed by elderly (age ≥65 years), labile PT-INR defined as episode(s) of PT-INR ≥3.5 during the study period, and antiplatelet use in patients with NVAF. For ICH, it was labile PT-INR (OR 5.00, 95% CI 2.39–9.66) followed by prior bleeding and antiplatelet use [25]. Although hypertension was not identified as an independent factor for major hemorrhage or ICH in this sub analysis [25], it was significant risk factor for major hemorrhage in another sub analysis on hypertension (HR 1.52, 95%CI 1.05–2.21) in patients with NVAF [31]. As on the AF type, the results on hypertension as an event risk often differ among studies. Possible reasons for the discrepant results include the different definition of hypertension was used among studies, that is, hypertension was defined as only the baseline systolic BP ≥140 mmHg in the former sub analysis [25], whereas as the baseline systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, history of hypertension, and/or under treatment in the latter subanalysis [31]. On the other hand, when BP values at the time closest to the event or at the end of follow-up were used for analysis, the systolic BP ≥136 mmHg was a strong risk factor for ICH (OR 4.55, 95%CI 1.89–10.96) even after adjusting for the components of the HAS-BLED score [31]. According to the subanalyses on hypertension of the phase III trials using NOACs [61-63,105,106], and the subanalyses of the Japanese AF registries [31,64], uncontrolled hypertension is also a certain risk factor for hemorrhagic events.
Valvular atrial fibrillation
Although NOACs can be used for the prevention of thromboembolism in patients with NVAF, these are contraindicated for patients with valvular AF including mechanical prosthetic valves and mitral stenosis. Since the effectiveness or safety of NOACs for valvular AF has not been proven [107,108], warfarin is still necessary for patients with valvular AF. In the J-RHYTHM Registry, 421 patients had valvular AF defined as mitral stenosis and/or mechanical valve replacement, accounting for 5.3% of the entire patients with AF, and they were analyzed separately from those with NVAF. Composite event of thromboembolism and major hemorrhage was less frequent in the PT-INR of 1.6–2.6 in patients with valvular AF [20], as well as those with NVAF [14], although the PT-INR of 2.0–3.0 is currently recommended [42].
Effects of Non-Vitamin K Antagonist Oral Anticoagulants
Although warfarin was the solo anticoagulant in the J-RHYTHM Registry because no NOAC had been approved in 2009 [13], the use of NOACs has increased in number during the extended follow-up period between 2012 and 2014 in the J-RHYTHM Registry 2 [39] (Figure 6). Consequently, the risk of thromboembolism in NOAC users was significantly lower than that in warfarin users (1.4±1.1 vs. 1.7±1.2 in CHADS2 score). All event rates of thromboembolism, major hemorrhage, and all-cause death in 923 patients receiving NOACs at the end of follow-up were significantly lower than those receiving warfarin even after adjusting multiple confounding factors. However, the follow-up period after initiating an anticoagulant was not considered, which was deemed as a limitation of this study [39]. On the other hand, in the Fushimi AF Registry, the first NOAC dabigatran was prescribed in 2.1% of patients at entry [43], and the use of NOACs gradually increased in number with various changing patterns during the follow-up period [109]. Considering the changes in anticoagulant status as a time-dependent covariate, NOAC use was not associated with stroke/systemic embolism or major bleeding events [109]. Subsequently, in the SAKURA AF Registry, which is another Japanese registry study in patients with NVAF receiving anticoagulation therapy, initiated in September 2013 [110], the incidence of thromboembolism and all-cause death in NOAC users was comparable to that in warfarin users, whereas major hemorrhage in NOAC users was less frequent than that in warfarin users after adjusting patient characteristics and duration of treatment using propensity score matching [111]. Affecting factors for the discrepant results on the effects of NOACs among these registries may include the differences in patient characteristics, rates of NOAC use, duration of medications, and changing patterns in anticoagulants. Further investigation may be necessary to clarify the efficacy and safety of NOACs compared with VKAs in real-world clinical setting.
Long-Term Effects of Warfarin
In the J-RHYTHM Registry 2, long-term event rates were also evaluated [39]. The 5-year incidence rates of thromboembolism and all-cause death were significantly lower in patients who continued warfarin therapy throughout the study period compared with those who did not receive any anticoagulant (5.0% vs. 7.8%, P=0.021 and 5.8% vs. 10.2%, P<0.001, respectively), whereas the incidence of major hemorrhage was higher in patients on warfarin therapy compared with those without anticoagulation therapy (5.9% vs. 2.6%, P=0.007) [39]. In addition, adjusted HRs for thromboembolism were significantly lower in all PT-INR subgroups, but those for major hemorrhage were significantly higher in the PT-INR of ≥2.6, compared with the no anticoagulation group as a reference, indicating that baseline PT-INR levels still influenced the incidence of subsequent events during the 5-year follow-up period. The beneficial effects of warfarin to prevent thromboembolism lasted for 5 years, whereas long-term use of warfarin might increase major hemorrhage [39]. These results support our previous reports on target PT-INR values in the J-RTYTHM Registry [14].
Perspectives and Direction of Registry Study
Although registry studies are not an RCT and thus have several limitations, they have played a substantial role in understanding the characteristics and the status of anticoagulation therapy in real-world patients with AF. However, several concerns to be resolved have also risen since event rates and effects of anticoagulants differed between registry studies.First, although the number of patients in the J-RHYTHM Registry was largest among single registry studies of AF in Japan, it was smaller than the other registries using big data such as insurance database in Western countries or nationwide registries in Taiwan [112], Sweden [55], and other countries. Furthermore, in the J-RHYTHM Registry, there was lacking in data on echocardiographic findings (left atrial size, left ventricular ejection faction, flow velocity) and biomarkers (brain natriuretic peptide, d-dimer, and others) that might be associated with adverse events. To resolve these concerns, the J-RISK AF Research Group attempted to identify the risk factors for adverse events in patients with AF using a larger database combined with 5 major Japanese AF registries including the J-RHYTHM Registry [75]. Consequently, age ≥75 years, hypertension, previous stroke, persistent or permanent AF, and low BMI of <18.5 kg/m2 were identified as risk factors for ischemic stroke in 12,289 Japanese patients with NVAF [75].
Another problem is that the causes of death are not always cardiogenic stroke or bleeding complications [36,57,113,114]. Non-cardiovascular death caused by infection or malignancy is reportedly more common in real-world clinical setting; 35.8% in the international GARFIELD-AF (Global Anticoagulant Registry in the Field-Atrial Fibrillation) [113], 42.7% in a French regional registry [114], and 66.0% in a French nationwide database [57], specifically in older patients [115]. So far, the target of risk assessment has mainly been for thromboembolism and major hemorrhage and the strategy of anticoagulation therapy has been determined by the net clinical benefit [10,28] based on both event risks. However, it will be necessary to consider mortality, to evaluate risks of death, and to determine a therapeutic strategy based on the net clinical outcome considering all-cause death. Certainly, low BMI [29], renal impairment [33], and anemia [37] were strongly associated with all-cause death and composite events including all-cause death in addition to older age, heart failure, prior stroke or TIA, and coronary artery disease in the J-RHYTHM Registry. Furthermore, other investigators have reported that frailty is also associated with worse prognosis in patients with AF; patients with frailty are less likely to receive anticoagulation therapy and have various comorbidities predisposing to adverse events [116-118]. Therefore, clinical factors not including conventional risk scores such as low BW or low BMI, renal impairment, anemia, and frailty could be more important to assess the total risk of patients with AF considering the prognosis of individuals. Since most of these factors are fit to the exclusion criteria in RCTs, it is difficult to validate these risk factors for adverse events in RCT, whereas it could be clarified in registry studies using real-world patients. Thus, registry study deserves to be achieved beyond evidence level.
Conclusions
In a recent decade, anticoagulation therapy in patients with AF has been shifted from warfarin to NOACs. Although information of NOACs is limited in the J-RHYTHM Registry, this registry study has provided the insights into the optimal PT-INR levels in patients receiving warfarin and into the risk assessment not only for thromboembolism and major hemorrhage but also for all-cause death. We believe that these findings could be helpful for all physicians in the management of Japanese patients with AF.
Acknowledgements
We would like to thank all investigators of the J-RHYTHM Registry and J-RHYTHM Registry 2 listed in the references [12], [13], [15], and [31]. Complete members of this study are shown in the Appendix.
Source of Funding
The J-RHYTHM Registry is registered at the University Hospital Medicine Information Network (UMIN) Clinical Trials Registry (UMIN000001569) and was supported by a grant from the Japan Heart Foundation (12080025). The J-RHYTHM Registry 2 was supported by a grant from the Japan Heart Foundation (12110015).
Conflict of Interest
Dr. Kodani received remuneration from Daiichi-Sankyo, Bristol-Myers Squibb, and Ono Pharmaceutical; Dr. Inoue received remuneration from Daiichi-Sankyo, Bayer Healthcare, Boehringer Ingelheim, and Bristol-Myers Squibb; Dr. Atarashi received remuneration from Daiichi-Sankyo; Dr. Okumura received research funding from Boehringer Ingelheim and Daiichi-Sankyo and remuneration from Boehringer Ingelheim, Bayer Healthcare, Daiichi-Sankyo, and Pfizer; and Dr. Yamashita received research funding from Daiichi-Sankyo, Bayer Healthcare, and Bristol-Meyers Squibb and remuneration from Daiichi-Sankyo, Pfizer, Bayer Healthcare, Bristol-Myers Squibb, Toa Eiyo, and Ono Pharmaceutical.
References
1. Feinberg WM, Blackshear JL, Laupacis A, et al. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995; 155: 469-73.
2. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001; 285: 2370-5.
3. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006; 114: 119-25.
4. Ohsawa M, Okayama A, Sakata K, et al. Rapid increase in estimated number of persons with atrial fibrillation in Japan: an analysis from national surveys on cardiovascular diseases in 1980, 1990 and 2000. J Epidemiol. 2005; 15: 194-6.
5. Inoue H, Fujiki A, Origasa H, et al. Prevalence of atrial fibrillation in the general population of Japan: an analysis based on periodic health examination. Int J Cardiol. 2009; 137: 102-7.
6. Wolf PA, Abbott RD, Kannel WB, et al. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991; 22: 983-8.
7. Atrial Fibrillation Investigators. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994; 154: 1449-57.
8. Hart RG, Pearce LA, Aguilar MI, et al. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007; 146: 857-67.
9. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010; 138: 1093-100.
10. Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009; 151: 297-305.
11. O'Leary CP, Cavender MA. Emerging opportunities to harness real world data: An introduction to data sources, concepts, and applications. Diabetes Obes Metab. 2020; 22 Suppl 3: 3-12.
12. Atarashi H, Inoue H, Okumura K, et al. Investigation of optimal anticoagulation strategy for stroke prevention in Japanese patients with atrial fibrillation -The J-RHYTHM Registry study design. J Cardiol. 2011; 57: 95-9.
13. Atarashi H, Inoue H, Okumura K, et al. Present status of anticoagulation treatment in Japanese patients with atrial fibrillation -A report from the J-RHYTHM Registry. Circ J. 2011; 75: 1328-33.
14. Inoue H, Okumura K, Atarashi H, et al. Target international normalized ratio values for preventing thromboembolic and hemorrhagic events in Japanese patients with non-valvular atrial fibrillation: results of the J-RHYTHM Registry. Circ J. 2013; 77: 2264-70.
15. J-RHYTHM Registry Investigators. Determinants of warfarin use and international normalized ratio levels in atrial fibrillation patients in Japan - Subanalysis of the J-RHYTHM Registry. Circ J. 2011; 75: 2357-62.
16. Inoue H, Atarashi H, Okumura K, et al. Impact of gender on the prognosis of patients with nonvalvular atrial fibrillation. Am J Cardiol. 2014; 113: 957-62.
17. Okumura K, Inoue H, Atarashi H, et al. Validation of CHA2DS2-VASc and HAS-BLED scores in Japanese patients with nonvalvular atrial fibrillation: an analysis of the J-RHYTHM Registry. Circ J. 2014; 78: 1593-9.
18. Inoue H, Atarashi H, Okumura K, et al. Thromboembolic events in paroxysmal vs. permanent non-valvular atrial fibrillation. Subanalysis of the J-RHYTHM Registry. Circ J. 2014; 78: 2388-93.
19. Yamashita T, Inoue H, Okumura K, et al. Warfarin anticoagulation intensity in Japanese nonvalvular atrial fibrillation patients: A J-RHYTHM Registry analysis. J Cardiol. 2015; 65: 175-7.
20. Kodani E, Atarashi H, Inoue H, et al. Target intensity of anticoagulation with warfarin in Japanese patients with valvular atrial fibrillation: Subanalysis of the J-RHYTHM Registry. Circ J. 2015; 79: 325-30.
21. Suzuki S, Yamashita T, Okumura K, et al. Incidence of ischemic stroke in Japanese patients with atrial fibrillation not receiving anticoagulation therapy - pooled analysis of the Shinken Database, J-RHYTHM Registry, and Fushimi AF Registry. Circ J. 2015; 79: 432-8.
22. Chishaki A, Kumagai N, Takahashi N, et al. Non-valvular atrial fibrillation patients with low CHADS2 scores benefit from warfarin therapy according to propensity score matching subanalysis using the J-RHYTHM Registry. Thromb Res. 2015; 136: 267-73.
23. Ogawa H, Akao M, Suzuki S, et al. Antiplatelet therapy in Japanese patients with atrial fibrillation without oral anticoagulants: Pooled analysis of Shinken Database, J-RHYTHM registry and Fushimi AF registry. Int J Cardiol. 2015; 190: 344-6.
24. Tomita H, Okumura K, Inoue H, et al. Validation of risk scoring system excluding female sex from CHA2DS2-VASc in Japanese patients with nonvalvular atrial fibrillation - Subanalysis of the J-RHYTHM Registry. Circ J. 2015; 79: 1719-26.
25. Tomita H, Okumura K, Inoue H, et al. Assessment of risk factors for bleeding in Japanese patients with non-valvular atrial fibrillation receiving warfarin treatment: A subanalysis of the J-RHYTHM Registry. Int J Cardiol. 2015; 201: 308-10.
26. Kodani E, Atarashi H, Inoue H, et al. Use of warfarin in elderly patients with non-valvular atrial fibrillation -Subanalysis of the J-RHYTHM Registry-. Circ J. 2015; 79: 2345-52.
27. Kodani E, Atarashi H, Inoue H, et al. Secondary prevention of stroke with warfarin in patients with non-valvular atrial fibrillation: Subanalysis of the J-RHYTHM Registry. J Stroke Cerebrovasc Dis. 2016; 25: 585.
28. Watanabe E, Yamamoto M, Kodama I, et al. Net clinical benefit of adding aspirin to warfarin in patients with atrial fibrillation: Insights from the J-RHYTHM Registry. Int J Cardiol. 2016; 212: 311-7.
29. Inoue H, Kodani E, Atarashi H, et al. Impact of body mass index on the prognosis of Japanese patients with non-valvular atrial fibrillation. Am J Cardiol. 2016; 118: 215-21.
30. Inoue H, Atarashi H, Kodani E, et al. Regional differences in frequency of warfarin therapy and thromboembolism in Japanese patients with non-valvular atrial fibrillation- Analysis of the J-RHYTHM Registry. Circ J. 2016; 80: 1548-55.
31. Kodani E, Atarashi H, Inoue H, et al. Impact of blood pressure control on thromboembolism and major hemorrhage in patients with nonvalvular atrial fibrillation: A subanalysis of the J-RHYTHM Registry. J Am Heart Assoc. 2016; 5: e004075.
32. Kumagai N, Nusser JA, Inoue H, et al. Effect of addition of a statin to warfarin on thromboembolic events in Japanese patients with nonvalvular atrial fibrillation and diabetes mellitus. Am J Cardiol. 2017; 120: 230-5.
33. Kodani E, Atarashi H, Inoue H, et al. Impact of creatinine clearance on outcomes in patients with non-valvular atrial fibrillation: a subanalysis of the J-RHYTHM Registry. Eur Heart J Qual Care Clin Outcomes. 2018; 4: 59-68.
34. Inoue H, Kodani E, Atarashi H, et al. Renal dysfunction affects anticoagulation control with warfarin and outcomes in Japanese elderly patients with non-valvular atrial fibrillation. Circ J. 2018; 82: 2277-83.
35. Inoue H, Kodani E, Atarashi H, et al. Time in therapeutic range and disease outcomes in elderly Japanese patients with nonvalvular atrial fibrillation. Circ J. 2018; 82: 2510-7.
36. Kodani E, Inoue H, Atarashi H, et al. Impact of digitalis use on mortality in Japanese patients with non-valvular atrial fibrillation- A subanalysis of the J-RHYTHM Registry. Circ J. 2019; 83: 1644-52.
37. Kodani E, Inoue H, Atarashi H, et al. Impact of hemoglobin concentration and platelet count on outcomes of patients with non-valvular atrial fibrillation: A subanalysis of the J-RHYTHM Registry. Int J Cardiol. 2020; 302: 81-7.
38. Kodani E, Inoue H, Atarashi H, et al. Predictive ability of creatinine clearance versus estimated glomerular filtration rate for outcomes in patients with non-valvular atrial fibrillation: Subanalysis of the J-RHYTHM Registry. Int J Cardiol Heart Vasc. 2020; 29: 100559.
39. Kodani E, Atarashi H, Inoue H, et al. Beneficial effect of non-vitamin K antagonist oral anticoagulants in patients with nonvalvular atrial fibrillation- Results of the J-RHYTHM Registry 2. Circ J. 2016; 80: 843-51.
40. Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001; 285: 2864-70.
41. Rosendaal FR, Cannegieter SC, van der Meer FJ, et al. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993; 69: 236-9.
42. JCS Joint Working Group. Guidelines for pharmacotherapy of atrial fibrillation (JCS 2013): Digest version. Circ J. 2014; 78: 1997-2021.
43. Akao M, Chun YH, Wada H, et al. Current status of clinical background of patients with atrial fibrillation in a community-based survey: the Fushimi AF Registry. J Cardiol. 2013; 61: 260-6.
44. Akao M, Chun YH, Esato M, et al. Inappropriate use of oral anticoagulants for patients with atrial fibrillation. Circ J. 2014; 78: 2166-72.
45. White HD, Gruber M, Feyzi J, et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med. 2007; 167: 239-45.
46. Masaki N, Suzuki M, Matsumura A, et al. Quality of warfarin control affects the incidence of stroke in elderly patients with atrial fibrillation. Intern Med. 2010; 49: 1711-6.
47. Morgan CL, McEwan P, Tukiendorf A, et al. Warfarin treatment in patients with atrial fibrillation: observing outcomes associated with varying levels of INR control. Thromb Res. 2009; 124: 37-41.
48. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010; 137: 263-72.
49. Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012; 33: 2719-47.
50. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS: The Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESCEndorsed by the European Stroke Organisation (ESO). Eur Heart J. 2016; 37: 2893-962.
51. Ogawa S, Aonuma K, Tse HF, et al. The APHRS's 2013 statement on antithrombotic therapy of patients with nonvalvular atrial fibrillation. J Arrhythm. 2013; 29: 190-20.
52. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014; 64: e1-76.
53. Chiang CE, Okumura K, Zhang S, et al. 2017 consensus of the Asia Pacific Heart Rhythm Society on stroke prevention in atrial fibrillation. J Arrhythm. 2017; 33: 345-67.
54. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019; 74: 104-32.
55. Friberg L, Rosenqvist M, Lip GY, et al. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012; 33: 1500-10.
56. Stroke Prevention in Atrial Fibrillation Investigators. Risk factors for thromboembolism during aspirin therapy in patients with atrial fibrillation: The stroke prevention in atrial fibrillation study. J Stroke Cerebrovasc Dis. 1995; 5: 147-57.
57. Fauchier L, Villejoubert O, Clementy N, et al. Causes of death and influencing factors in patients with atrial fibrillation. Am J Med. 2016; 129: 1278-87.
58. Magnani JW, Rienstra M, Lin H, et al. Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation. 2011; 124: 1982-93.
59. Iguchi M, Tezuka Y, Ogawa H, et al. Incidence and risk factors of stroke or systemic embolism in patients with atrial fibrillation and heart failure- The Fushimi AF Registry. Circ J. 2018; 82: 1327-35.
60. D'Agostino RB, Wolf PA, Belanger AJ, et al. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994; 25: 40-3.
61. Vemulapalli S, Hellkamp AS, Jones WS, et al. Blood pressure control and stroke or bleeding risk in anticoagulated patients with atrial fibrillation: Results from the ROCKET AF Trial. Am Heart J. 2016; 178: 74-84.
62. Rao MP, Halvorsen S, Wojdyla D, et al. Blood pressure control and risk of stroke or systemic embolism in patients with atrial fibrillation: Results from the apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) Trial. J Am Heart Assoc. 2015; 4: e002015.
63. Nagarakanti R, Wallentin L, Noack H, et al. Comparison of characteristics and outcomes of dabigatran versus warfarin in hypertensive patients with atrial fibrillation (from the RE-LY Trial). Am J Cardiol. 2015; 116: 1204-9.
64. Ishii M, Ogawa H, Unoki T, et al. Relationship of hypertension and systolic blood pressure with the risk of stroke or bleeding in patients with atrial fibrillation: The Fushimi AF Registry. Am J Hypertens. 2017; 30: 1073-82.
65. Proietti M, Romiti GF, Olshansky B, et al. Systolic blood pressure visit-to-visit variability and major adverse outcomes in atrial fibrillation: The AFFIRM Study (Atrial Fibrillation Follow-up Investigation of Rhythm Management). Hypertension. 2017; 70: 949-58.
66. Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011; 342: d124.
67. Pearce LA, Hart RG, Halperin JL, et al. Assessment of three schemes for stratifying stroke risk in patients with nonvalvular atrial fibrillation. Am J Med. 2000; 109: 45-51.
68. Stroke Prevention in Atrial Fibrillation Investigators. Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet. 1996; 348: 633-8.
69. Inoue H, Atarashi H. Risk factors for thromboembolism in patients with paroxysmal atrial fibrillation. Am J Cardiol. 2000; 86: 852-5.
70. Olesen JB, Fauchier L, Lane DA, et al. Risk factors for stroke and thromboembolism in relation to age among patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. Chest. 2012; 141: 147-53.
71. Friberg L, Benson L, Rosenqvist M, et al. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ. 2012; 344: e3522.
72. Nielsen PB, Skjoth F, Overvad TF, et al. Female sex Is a risk modifier rather than a aisk factor for stroke in atrial fibrillation: Should We use a CHA2DS2-VA Score rather than CHA2DS2-VASc? Circulation. 2018; 137: 832-40.
73. Hohnloser SH, Pajitnev D, Pogue J, et al. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W Substudy. J Am Coll Cardiol. 2007; 50: 2156-61.
74. Takabayashi K, Hamatani Y, Yamashita Y, et al. Incidence of stroke or systemic embolism in paroxysmal versus sustained atrial fibrillation: The Fushimi Atrial Fibrillation Registry. Stroke. 2015; 46: 3354-61.
75. Okumura K, Tomita H, Nakai M, et al. Risk factors associated with ischemic stroke in Japanese patients with nonvalvular atrial fibrillation. JAMA Netw Open. 2020; 3: e202881.
76. Wang J, Yang YM, Zhu J, et al. Overweight is associated with improved survival and outcomes in patients with atrial fibrillation. Clin Res Cardiol. 2014; 103: 533-42.
77. Wang HJ, Si QJ, Shan ZL, et al. Effects of body mass index on risks for ischemic stroke, thromboembolism, and mortality in Chinese atrial fibrillation patients: a single-center experience. PLoS One. 2015; 10: e0123516.
78. Ardestani A, Hoffman HJ, Cooper HA, et al. Obesity and outcomes among patients with established atrial fibrillation. Am J Cardiol. 2010; 106: 369-73.
79. Overvad TF, Rasmussen LH, Skjoth F, et al. Body mass index and adverse events in patients with incident atrial fibrillation. Am J Med. 2013; 126: 640 e9-17.
80. Badheka AO, Rathod A, Kizilbash MA, et al. Influence of obesity on outcomes in atrial fibrillation: yet another obesity paradox. Am J Med. 2010; 123: 646-51.
81. Lavie CJ, Milani RV, Ventura HO, et al. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009; 53: 1925-32.
82. Vemmos K, Ntaios G, Spengos K, et al. Association between obesity and mortality after acute first-ever stroke: the obesity-stroke paradox. Stroke. 2011; 42: 30-6.
83. Hamatani Y, Ogawa H, Uozumi R, et al. Low body weight is associated with the incidence of stroke in atrial fibrillation patients - Insight from the Fushimi AF Registry. Circ J. 2015; 79: 1009-17.
84. Go AS, Fang MC, Udaltsova N, et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 2009; 119: 1363-9.
85. Olesen JB, Lip GY, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012; 367: 625-35.
86. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004; 351: 1296-305.
87. Nakayama M, Metoki H, Terawaki H, et al. Kidney dysfunction as a risk factor for first symptomatic stroke events in a general Japanese population--the Ohasama study. Nephrol Dial Transplant. 2007; 22: 1910-5.
88. Abe M, Ogawa H, Ishii M, et al. Relation of stroke and major bleeding to creatinine clearance in patients with atrial fibrillation (from the Fushimi AF Registry). Am J Cardiol. 2017; 119: 1229-37.
89. Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005; 67: 2089-100.
90. Kwok CS, Tiong D, Pradhan A, et al. Meta-Analysis of the Prognostic Impact of Anemia in Patients Undergoing Percutaneous Coronary Intervention. Am J Cardiol. 2016; 118: 610-20.
91. Numasawa Y, Ueda I, Sawano M, et al. Relation of Baseline Hemoglobin Level to In-Hospital Outcomes in Patients Who Undergo Percutaneous Coronary Intervention (from a Japanese Multicenter Registry). Am J Cardiol. 2018; 121: 695-702.
92. Nagatomo Y, Yoshikawa T, Okamoto H, et al. Anemia is associated with blunted response to beta-blocker therapy using carvedilol- Insights from Japanese Chronic Heart Failure (J-CHF) Study. Circ J. 2018; 82: 691-8.
93. Sharma S, Gage BF, Deych E, et al. Anemia: an independent predictor of death and hospitalizations among elderly patients with atrial fibrillation. Am Heart J. 2009; 157: 1057-63.
94. Hamatani Y, Yamashita Y, Esato M, et al. Predictors for stroke and death in non-anticoagulated Asian patients with atrial fibrillation: The Fushimi AF Registry (doi: 10.1371/journal.pone.0142394.). PLoS One. 2015; 10: e0142394.
95. Park J, Cha MJ, Choi YJ, et al. Prognostic efficacy of platelet count in patients with nonvalvular atrial fibrillation. Heart Rhythm. 2019; 16: 197-203.
96. An Y, Ogawa H, Esato M, et al. Cardiovascular events and mortality in patients with atrial fibrillation and anemia (from the Fushimi AF Registry). Am J Cardiol. 2020.
97. Petersen P, Boysen G, Godtfredsen J, et al. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Lancet. 1989; 333: 175-9.
98. Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011; 364: 806-17.
99. Sato H, Ishikawa K, Kitabatake A, et al. Low-dose aspirin for prevention of stroke in low-risk patients with atrial fibrillation: Japan Atrial Fibrillation Stroke Trial. Stroke. 2006; 37: 447-51.
100. Ho CW, Ho MH, Chan PH, et al. Ischemic stroke and intracranial hemorrhage with aspirin, dabigatran, and warfarin: impact of quality of anticoagulation control. Stroke. 2015; 46: 23-30.
101. Whitbeck MG, Charnigo RJ, Khairy P, et al. Increased mortality among patients taking digoxin--analysis from the AFFIRM study. Eur Heart J. 2013; 34: 1481-8.
102. Lopes RD, Rordorf R, De Ferrari GM, et al. Digoxin and mortality in patients with atrial fibrillation. J Am Coll Cardiol. 2018; 71: 1063-74.
103. Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011; 123: 2363-72.
104. Inoue H, Uchiyama S, Atarashi H, et al. Effectiveness and safety of long-term dabigatran among patients with non-valvular atrial fibrillation in clinical practice: J-Dabigatran Surveillance. J Cardiol. 2019; 73: 507-14.
105. Lip GY, Frison L, Grind M, et al. Effect of hypertension on anticoagulated patients with atrial fibrillation. Eur Heart J. 2007; 28: 752-9.
106. Park S, Bergmark BA, Shi M, et al. Edoxaban Versus Warfarin Stratified by Average Blood Pressure in 19 679 Patients With Atrial Fibrillation and a History of Hypertension in the ENGAGE AF-TIMI 48 Trial. Hypertension. 2019; 74: 597-605.
107. Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013; 369: 1206-14.
108. De Caterina R, John Camm A. Non-vitamin K antagonist oral anticoagulants in atrial fibrillation accompanying mitral stenosis: the concept for a trial. Europace. 2016; 18: 6-11.
109. Yamashita Y, Uozumi R, Hamatani Y, et al. Current status and outcomes of direct oral anticoagulant use in real-world atrial fibrillation patients- Fushimi AF Registry. Circ J. 2017; 81: 1278-85.
110. Okumura Y, Yokoyama K, Matsumoto N, et al. Current use of direct oral anticoagulants for atrial fibrillation in Japan: Findings from the SAKURA AF Registry. J Arrhythm. 2017; 33: 289-96.
111. Okumura Y, Yokoyama K, Matsumoto N, et al. Three-Year Clinical Outcomes Associated With Warfarin vs. Direct Oral Anticoagulant Use Among Japanese Patients With Atrial Fibrillation- Findings From the SAKURA AF Registry. Circ J. 2018; 82: 2500-9.
112. Chao TF, Liu CJ, Tuan TC, et al. Rate-control treatment and mortality in atrial fibrillation. Circulation. 2015; 132: 1604-12.
113. Bassand JP, Accetta G, Camm AJ, et al. Two-year outcomes of patients with newly diagnosed atrial fibrillation: results from GARFIELD-AF. Eur Heart J. 2016; 37: 2882-9.
114. Fauchier L, Samson A, Chaize G, et al. Cause of death in patients with atrial fibrillation admitted to French hospitals in 2012: a nationwide database study. Open Heart. 2015; 2: e000290.
115. An Y, Ogawa H, Yamashita Y, et al. Causes of death in Japanese patients with atrial fibrillation: The Fushimi Atrial Fibrillation Registry. Eur Heart J Qual Care Clin Outcomes. 2019; 5: 35-42.
116. Perera V, Bajorek BV, Matthews S, et al. The impact of frailty on the utilisation of antithrombotic therapy in older patients with atrial fibrillation. Age Ageing. 2009; 38: 156-62.
117. Nguyen TN, Cumming RG, Hilmer SN, et al. The impact of frailty on mortality, length of stay and re-hospitalisation in older patients with atrial fibrillation. Heart Lung Circ. 2016; 25: 551-7.
118. Wilkinson C, Todd O, Clegg A, et al. Management of atrial fibrillation for older people with frailty: a systematic review and meta-analysis. Age Ageing. 2019; 48: 196-203.
Received: Sep 23, 2020 ;
Accepted: Sep 30, 2020;
Published: Nov 06, 2020 .
To cite this article : Kodani E, Atarashi H, Inoue H, et.al. Anticoagulation Therapy and Event Risk in Japanese Patients with Atrial Fibrillation: Insight from the J-RHYTHM Registry. British Heart Diseases. 2020; 2:1.
© Kodani E, et.al. 2020.
