Research/ Open Access
DOI: 10.31488 /bjhd.116
A Randomized Clinical Trial about the Use of Intravenous Iron in Cardiac Surgery: Results of Three Analysis
Miguel Ángel Pico-Picos1, Pilar Garrido-Martín2, Patricia Rodríguez-Fortúnez3, Alejandro Jiménez-Sosa4, Javier Montoto- López2, Rafael Martínez-Sanz2
1.Department of Clinical Laboratory, Hospital Universitario de Canarias, Tenerife, Spain
2.Department of Cardiovascular Surgery, Hospital Universitario de Canarias, Tenerife, Spain
3.Clinical Trials Unit, Department of Pharmacology, Hospital Universitario de Canarias, Tenerife, Spain
4. Research Unit, Hospital Universitario de Canarias, Tenerife, Spain
*Corresponding author: Pilar Garrido-Martín PhD, Servicio de Cirugía Cardiovascular, Hospital Universitario de Canarias,
Ofra s/n, La Laguna, 38320. Tenerife. Canary Islands, Spain, Tel: + 34 922 678 000
Patricia Rodríguez-Fortúnez PhD, Clinical Trials Unit, Servicio de Farmacología Clínica, Hospital Universitario de Canarias,
Ofra s/n, La Laguna, 38320. Tenerife, Canary Islands, Spain, Tel: + 34 922 678 000
Abstract
Objective: The incidence of postoperative anemia is high in cardiac surgery, with bleeding being the main cause. Iron therapy is one of the most common empirical treatment strategies. In our study we evaluated the efficacy of oral and intravenous iron administration to correct anemia, as well as the impact on transfusion requirements and postoperative hospital stay in patients undergoing cardiac surgery. Methods: Prospective, adaptive, randomized, double blind, placebo controlled clinical trial with three parallel groups. Group I (n=72): 100 mg intravenous iron hydroxide complex III-sucrose/24 h preoperatively and 1 tablet/24 h oral placebo pre- and postoperatively up to 1 month after discharge. Group II (n=73): intravenous placebo/24h preoperatively and 1 tablet /24 h of 105 mg oral iron II sulphate pre and postoperatively until 1 month after discharge. Group III (n=65): intravenous placebo/24 h preoperatively and 1 tablet/24 h oral placebo pre and postoperatively until 1 month after discharge. Results: The baseline clinical and demographic characteristics were similar among the three groups. We did not find differences between the groups studied in Hb levels during the postoperative period. Serum ferritin levels were increased in patients of group I both at hospital discharge and at the follow-up visit (p <0.001). We did not see differences regarding the transfusion requirements and the postoperative hospital stay either. Conclusions: We could not to demonstrate the effectiveness of the administration of iron to correct the postoperative anemia in non-iron deficient patients after cardiac surgery, nor the reduction of transfusion requirements and nor the postoperative hospital stay.
Keywords: Cardiac surgery, Intravenous iron supplement, anemia, transfusion
Introduction
The incidence of postoperative anemia is high in cardiac surgery [1]. Its origin is multifactorial, with bleeding being the main cause, often requiring the use of packed red blood cells to correct it [2]. Hyperinflammation causes impaired bone marrow function by means of blunted erythropoietin response, reduced iron availability.
Iron is essential for erythropoiesis [3, 4] being the mobilization speed from its storage places one of the limiting factors [5].
The effectiveness of oral iron therapy is limited by its low bioavailability and intolerance that many patients present. The use of intravenous iron preparations has been positioned as an alternative [6, 7].
It has been demonstrated that intravenous iron therapy is effective, both in the reduction of transfusion requirements and in the elevation of hemoglobin levels, in some clinical specialties (nephrology, oncology, hematology, cardiology….) and surgical specialties (general surgery, orthotrauma, gynecology, obstetrics...) [8]. In cardiac surgery its efficacy is controversial [9, 10].
Objectives
To estimate the efficacy of oral and intravenous iron versus placebo in correcting postoperative anemia in patients undergoing cardiac surgery with cardiopulmonary bypass (CBP), by evaluating hemoglobin (Hb) values using analysis per protocol (PP), by intention to treat (ITT) and modified intention to treat (mITT). Secondary objectives were to assess whether iron administration decreases transfusion needs and postsurgical hospital stay.
Performing the trial by the three methods of analysis, seeking to enhance the results of the study and reduce bias.
Methods
Ethical statement
The committee of ethics and clinical research of the University Hospital of the Canary Islands approved the study on 30th of March 2006. The internal study ID asigned was: iron treatment / 2006.
This study was also approved by the Spanish National Agency for Medicines and Health Products. The ClinicalTrials.gov Identifier assigned was NCT01078818.
All patients included in the trial adequately signed the informed consent.
Study design
(ClinicalTrials.gov number NCT01078818) An adaptive study [11] in patients undergoing cardiac surgery with CBP randomly assigned to three groups: Group I patients treated pre- and postoperatively with an intravenous complex of iron hydroxide III-sucrose (Venofer®; Uriach Laboratory), Group II patients treated pre and postoperatively orally with iron sulphate II (Ferogradumet®, Abbott Laboratory) and Group III patients treated pre and postoperatively with oral and intravenous placebo. The randomization of patients was carried out using an excel table of random numbers. This study was performed at the Hospital Universitario de Canarias, Spain.
We included patients older than 18 years-old, elective cardiac surgery under CBP, without anemia, and signing informed consent. Anemia was defined according to the World Health Organization (WHO) definition (Hb <13 g/dL for males and <12 g/dL for females). The exclusion criteria were: cardiac surgery without CBP or emergency surgery, without fibrinolytic treatment in the 48 hours prior to surgery, renal dysfunction (serum creatinine > 1.5 mg/dL), endocarditis surgery, re-operated patients, pregnancy, history of digestive hemorrhage, iron deficiency, vitamin B12 and/or folic acid, any hematological disease, asthma, liver disease, acute or chronic infection, allergy to iron and/or excipients.
The criteria for transfusing red blood cell (RBC) concentrates was the presence of low cardiac output syndrome associated with Hb levels < 8 g/dL and < 7 g/dL in patients undergoing coronary and valvular surgery, respectively.
Dosage of drugs
The treatment regimen for the 3 parallel groups of patients was the same; 1 diary dose of intravenous treatment (iron or placebo) for three days during preoperative and postoperative period combined with oral treatment (oral iron or placebo) during the same preoperative period and during the postoperative period, maintaining oral treatment for 1 month after hospital discharge. The oral iron treatment up was with iron II oral sulphate/24h, 1 tablet a day. In this way: Group I: Patients received pre and postoperatively 100 mg intravenous iron hydroxide complex III-sucrose/24h and 1 oral placebo tablet/24h. Group II: Patients were treated pre and postoperatively with intravenous placebo/24h and 1 tablet of 105 mg iron II oral sulphate/24h. Group III: Patients were treated pre and postoperatively with intravenous placebo/24 h and 1 tablet of oral placebo/24 h.
Table 1:Clinical demographic characteristics
| GROUP I | GROUP II | GROUP III | P value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ITT (n=72) | ITTv (n=68) | PP (n=53) | ITT (n=73) | ITTv (n=69) | PP (n=54) | ITT (n=65) | ITTv (n=63) | PP (n=52) | ITT | ITTv | PP | |
| Sex: n (%) | ||||||||||||
| Female | 23 (31.9) | 22 (32.4) | 16 (30.2) | 26 (35.6) | 23 (33.3) | 15 (27.8) | 19 (29.2) | 19 (30.2) | 12 (23.1) | 0.72 | 0.87 | 0.71 |
| Male | 49 (68.1) | 46 (67.6) | 37 (69.8) | 47 (64.4) | 46 (66.7) | 39 (72.2) | 46 (70.8) | 44 (69.8) | 40 (76.9) | |||
| Age (years) | 65 ± 12 | 65 ± 12 | 64 ± 11 | 67 ± 10 | 67 ± 9 | 66 ± 10 | 65 ± 12 | 65 ± 12 | 65 ± 12 | 0.84 | 0.84 | 0.81 |
| Parsonnet Score | 7.6 ± 5.10 | 7.6 ± 5.15 | 6.9 ± 4.71 | 6.7 ± 2.97 | 6.7 ± 3.02 | 6.7 ± 3.24 | 7.4 ± 5.34 | 7.4 ± 5.38 | 7.2 ± 4.82 | 0.79 | 0.68 | 0.98 |
| NYHA: n(%) (Functional class) | ||||||||||||
| Class I | 4 (5.6) | 4 (5.9) | 2 (3.8) | 2 (2.8) | 2 (2.9) | 1 (1.9) | 6 (9.2) | 6 (9.5) | 4 (7.7) | 0.35 | 0.21 | 0.24 |
| Class II | 29 (40.8) | 27 (39.7) | 22 (41.5) | 24 (33.3) | 21 (30.9) | 16 (30.2) | 30 (46.2) | 29 (46.0) | 26 (50.0) | |||
| Class III | 26 (36.6) | 25 (36.8) | 23 (43.4) | 33 (45.8) | 33 (48.5) | 30 (56.6) | 22 (33.8) | 22 (34.9) | 18 (34.6) | |||
| Class IV | 12 (16.9) | 12 (17.6) | 6 (11.3) | 13 (18.1) | 12 (17.6) | 6 (11.3) | 7 (10.8) | 6 (9.5) | 4 (7.7) | |||
| Heart disease: n(%) | ||||||||||||
| Coronary | 30 (41.7) | 28 (41.2) | 21 (39.6) | 27 (37.0) | 25 (36.2) | 21 (38.9) | 34 (52.3) | 34 (54.0) | 28 (53.8) | 0.02 | 0.02 | 0.05 |
| Valvular | 34 (47.2) | 33 (48.5) | 29 (54.7) | 36 (49.3) | 34 (49.3) | 24 (44.4) | 19 (29.2) | 18 (28.6) | 15 (28.8) | |||
| Mixed | 4 (5.6) | 4 (5.9) | 3 (5.7) | 10 (13.7) | 10 (14.5) | 9 (16.7) | 11 (16.9) | 10 (15.9) | 9 (17.3) | |||
| Other | 4 (5.6) | 3 (4.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.5) | 1 (1.6) | 0 (0.0) | |||
The administration of intravenous iron was carried out by diluting 100 mg in 200 ml of physiological saline solution with an infusion time of 1 hour. An infusion of 200 ml of saline was used as an intravenous placebo. The medication was prepared and provided by the Pharmacy Service following the list of randomized numbers, and neither the healthcare staff nor the patients knew the assigned treatment.
The treatment was given during hospital admission and postoperatively in the Intensive Care Unit (ICU) and / or hospital ward.
Procedure
The sociodemographic data, cardiovascular risk factors and necessary parameters to calculate the surgical risk were collected during the inclusion visit. The data of Hb (g/dL) and hematocrit (%), as main variables of assessment, were recorded at the time of hospital admission, in the operating room, at the time of admission to the intensive care unit (ICU), at the time of discharge from the ICU, at hospital discharge and during the first follow-up visit (one month after hospital discharge). The reticulocyte count data, expressed as a percentage of the total red blood cells, the reticulocyte index, (additional correction that takes into account the compensatory red cell stimulus that occurs in severe anemias) and the serum ferritin concentration (ng/mL), were collected at the time of admission, at hospital discharge and during the follow-up visit at consultation. The outpatient follow-up has been only once a month after discharge.
In addition, data on the amount of blood products used were obtained both during surgery and in the postoperative period.
Statistical analysis
The intention-to-treat (ITT) analysis (includes all randomized patients) aims to preserve the balance between the groups participating in the trial achieved through randomization, and thus minimize bias by admitting the lack of adherence to treatment, resembling more to reality that we find in daily clinical practice [12]. It is also recommended by expert groups and organizations in the methodology of clinical trials [13-15]. Its modified ITT variant (mITT) (excludes patients considered not selectable after randomization and patients who have not received any dose of treatment), given that some authors maintain that the inclusion of patients who did not receive treatment could bias the efficacy of the drug to study [16] and the per protocol (PP) analysis (includes randomized patients who were adherent to the protocol and followed periodically until the end of the trial).
Sample size
According with the adaptive nature of the design, [11] the sample size was calculated by performing an intermediate statistical analysis when 20 patients from each group had been included, estimated at 210 the number of patients needed to test the magnitude of the effect of iron supplementation on Hb levels. The mean hemoglobin in an exploratory analysis with the first 60 patients included showed a common standard deviation of 1.17 g/dL in hemoglobin between the placebo and oral iron groups (intergroup comparison). To demonstrate with a 95% confidence level and 80% power an expected increase of at least 0.48 g/dL in hemoglobin after ICU discharge in the oral iron group compared to the placebo group, 73 patients per group are needed (total n = 210). The sample size calculation was calculated for ITT analysis and using Grammo v. 7.12 ("Institut Municipal d'Investigació Mèdica", Barcelona, Spain).”
Data Analysis
The continuous variables are expressed with means and standard deviations and the categorical variables with frequencies and percentages. The comparison between groups of the quantitative variables was performed with two way analysis of variance with repeated measures including post hoc comparisons using the Scheffee test, and the Kruskal-Wallis and Mann-Whitney tests, as appropriate. The comparison between proportions was made using the chi-square or Fisher exact test. The non parametric correlation studies were performed using the Spearman correlation test.
Statistical analyses were performed using the SPSS v 17.0 program (SPSS Inc., Chicago, IL, USA) and Statistica V. 8.0. (StatSoft Tulsa, OK). Values of p < .05 were considered significant.
Results
We included 210 patients in the 2-year study (May 2007 - August 2009; first patient was enrolled on May 07th 2007 and last patient was enrolled on June 19th 2009) randomized in 3 parallel treatment groups. Figure 1 shows the CONSORT diagram of the study. 67.6% of these patients were male and 32.4% were women with a mean age of 66 ± 11 years. No differences were observed between the groups in the clinical and demographic characteristics and not differences in the estimation of surgical risk (Parsonnet Score) (Table 1).
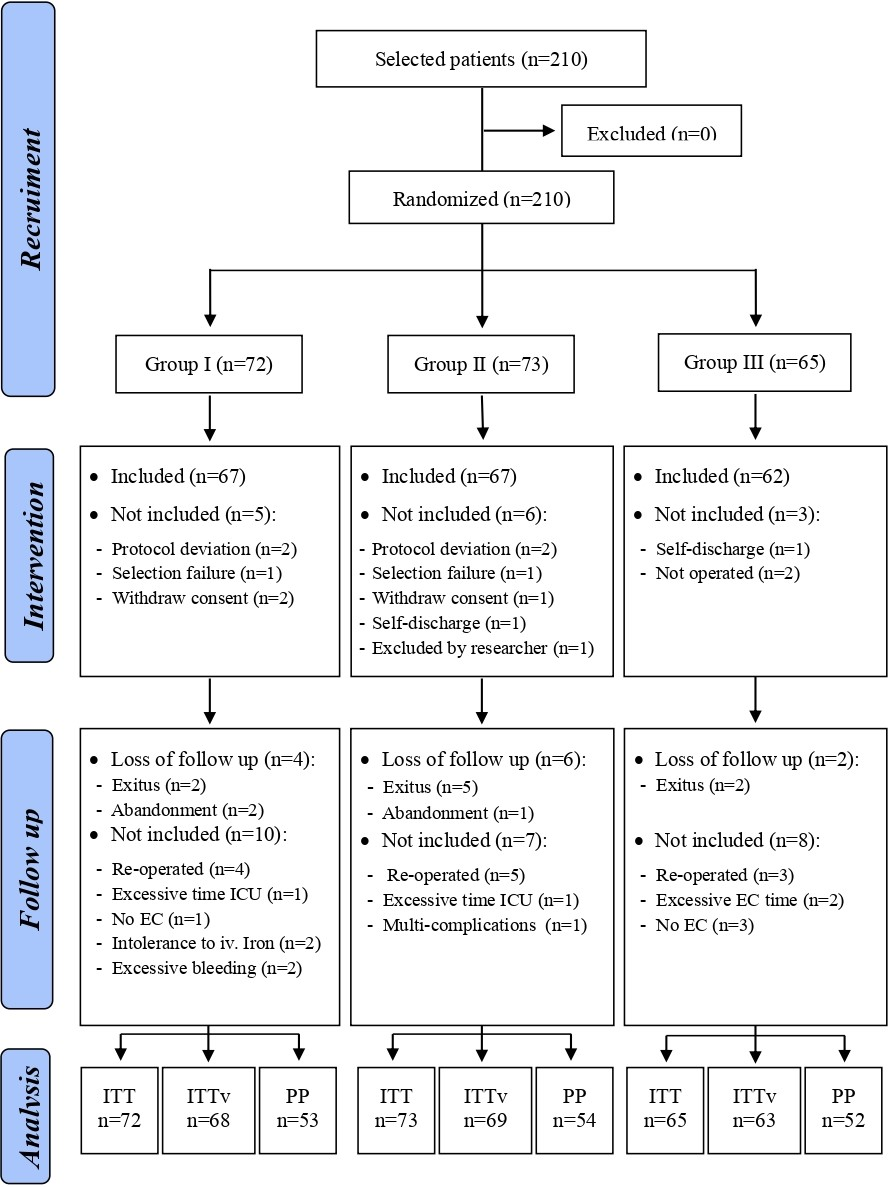
Figure 1:CONSORT diagram of the study
In the ITT analysis, first, there were no differences in Hb levels in the intergroups comparison (p = 0.91) nor the interaction between groups and repeated measures (p = 0.10). Second, there were no differences in absolute reticulocyte count in the intergroups comparison (p = 0.14) nor the interaction between groups and repeated measures (p = 0.99). And third, there were no differences in reticulocyte index in the intergroups comparison (p = 0.28) nor the interaction between groups and repeated measures (p = 0.90) (Supplementary table 1). On the other hand, serum ferritin levels showed a clear increase in group I patients at the time of hospital discharge, staying above the normal range at the time of the follow-up visit, with patients in groups II and III. These differences between the mean ferritin values showed statistical significance in the three analysis modalities (p< .001) in these 2 test points. The mean values of serum ferritin observed between the patients in group II and group III were similar both at discharge and during the follow-up visit in consultation (Supplementary table 1).
No differences were observed among the three groups in the three modalities of analysis in terms of the number of patients that required the transfusion of RBC, nor in the number of units of RBC required per patient, nor the distribution of patients depending on the units transfused in each treatment group, including in this last section also patients who did not require transfusion (Supplementary table 2).
The average postoperative hospital stay was greater in patients included in the ITT and mITT analyses compared to patients who strictly complied with the trial protocol. Although we did not find significant differences between the three groups of patients studied in terms of the average postoperative stay in each of the analysis strategies performed (Supplementary table 3).
In the per protocol analyses, significant differences were observed between the groups in length of stay after leaving the intensive care unit (p= 0.03). The stay was shorter in the group that received intravenous iron (Group I) compared to the placebo group (Group III). No differences were observed between Group II and Group III and Group I.
Discussion
The results obtained with respect to the main study variable indicate that the administration of iron, in intravenous or oral form, does not produce a greater recovery of Hb levels (primary outcome) in any of the control points throughout the trial, in any of the analysis modalities carried out (ITT, mITT and PP).
Our results are in line with two other randomized clinical trials (RCTs) in cardiac surgery [9, 17] that argue that intravenous iron administration, alone or in association with recombinant human erythropoietin (rhEPO), is ineffective in recovering hemoglobin levels. It should be noted that in these trials, the administration of the treatment was performed exclusively during the postoperative period. In contrast, in another placebo-controlled RCT in cardiac surgery [10] when a single dose of intravenous iron was administered (1000 mg) the day before surgery, a significantly higher Hb serum level was observed at 4 weeks after the intervention in the group of patients who received intravenous iron.
Cladellas et al. [18] in a prospective study administering 5 preoperative doses of intravenous iron associated with rhEPO, initiating treatment one month before surgery, observed a significant increase in Hb levels before surgery, when compared with patients in a study observational retrospective. However, at the end of the study, the Hb levels of the patients were similar in both studies. In our hospital setting, a longer treatment regimen cannot be performed on an outpatient basis and the preoperative period established has been the longest possible to avoid an excessive preoperative hospital stay and an unjustified delay in surgery.
The elevation of absolute reticulocyte figures is shown in other published trials with similar results to ours [9]. However, other authors [10, 17] did observe significant differences in patients treated with intravenous iron. This difference with reference to our results may be due to the fact that these studies determined the maximum peak in the reticulocyte count between days 5 and 7 after surgery, the different iron doses used [10] or the concomitant administration of rhEPO [17].
Ferritin levels increase rapidly, reaching a maximum peak at the time of hospital discharge, significantly decreasing in the consultation visit, maintaining the differences between patients in group I and groups II and III. These results are compatible with those found in similar studies in cardiac surgery [9, 10]. It should be noted that the ferritin figures between the groups that did not receive intravenous iron (II and III) are similar in these two moments of the trial, so the elevation that occurs at the time of hospital discharge could be due to the condition of reactant of acute phase of this molecule. In the follow-up visit it’s possible to see a decrease over 50% in serum ferritin levels.
The relationship between transfusion of RBC and the increased risk of postoperative infections, morbidity and mortality and length of hospital stay have been widely demonstrated [19, 20]. In our RCT, we found no significant differences between the three treatment groups, either in the number of patients requiring RBC transfusion or in the number of units required per patient. These results are in line with those found in other studies performed in cardiac surgery. [10, 17] On the contrary, there are many studies such as Theusinger,[21] Cuenca,[22] García-Erce [23] and Serrano-Trenas [24] in orthotrauma surgery and Okuyama [25] in colorectal surgery demonstrating that intravenous iron administration is effective in reducing the blood transfusion requirements of these patients.
The average postoperative stay was higher in the patients included in the analyses by ITT and mITT, regarding the stay that patients who strictly complied with the trial protocol presented. The mean stay in the ITT and mITT analyses was higher in groups I (intravenous iron) and III treatment (placebo). This was due to the fact that two patients from group I treatment presented postoperative multi-complications that required a very long stay in the ICU (75 and 80 days). On the other hand, there were two patients (1 patient in group I and another in group III) who presented anoxic encephalopathy and remained hospitalized for 237 and 535 days respectively, awaiting transfer to a chronic hospital.
Eliminating these patients from the analysis, because they were considered "outliers", the stay of patients in group I was slightly lower compared to patients in groups II and III (12.2 vs 14.2 vs 13.8 days in group I, II and III respectively), without reaching these differences statistical significance.
Our results are in agreement with those observed by other authors such as Karkouti [17] and Serrano-Trenas, [24] where the administration of iv iron associated or not, with erythropoiesis stimulating agents, does not reduce hospital stay. However, Cuenca [22] did observe a decrease in hospital stay in patients treated with intravenous iron in hip surgery. Currently, an international multicenter randomized study (https://clinicaltrials.gov/ct2/show/record/NCT02632760) is ongoing using i.v. iron (ferric carboxymaltose 1000 mg) during 1-10 weeks prior to surgery. The results of the trial will be relevant to improve the correction of anemia on cardiac surgery.
Study Limitations
One of the limitations of this study could be the short period of preoperative treatment, from 4 to 6 days, compared to other similar studies where the preoperative treatment is extended up to 1 month before surgery. In our environment at the time of the trial it was not possible to do otherwise.
Another limitation could be the assessment of Hb levels one month after hospital discharge. It is possible that this period of time is insufficient for a recovery of anemia after surgery. In any case, the intention was to evaluate the early recovery of anemia and the reduction of transfusion needs that are the relevant points from the clinical-surgical point of view.
Conclusions
It is evident that intravenous iron administration is effective in the restoration of iron reserves valued by the increase in ferritin levels, demonstrating the excellent bioavailability of this form of iron administration. Although we have not been able to demonstrate that the administration of intravenous iron improves the correction of postoperative anemia in non-iron deficient patients undergoing programmed cardiac surgery, assessed with hemoglobin levels at different times of the postoperative period.
We also did not find that the administration of intravenous iron is effective in reducing transfusion requirements or in reducing the total postoperative hospital stay of patients undergoing cardiac surgery with CBP.
The short-term iron treatment prior to surgery could be a limitation that can explain not to achieve a major difference between groups in terms of Hb recovering values.
Per Protocol analysis results with iv iron treatment presents the shortest stay and this could have relevant economic implications. But this point is not included in our main objective and it would be necessary to perform an economic analysis to confirm it. These results should be considered with caution due the limited sample size.
Based on the results, it would be advisable to perform more randomized trials with a greater number of patients and with treatment regimens with higher iron concentration or longer over time, to confirm these results and re-evaluate the efficacy of intravenous iron administration in non-anemic patients undergoing elective cardiac surgery.
Abbreviations
CBP: cardiopulmonary bypass, Hb: hemoglobin, HUC: Hospital Universitario de Canarias, ICU: intensive care unit, ITT: intention to treat, PP: per protocol, mITT: modified intention to treat, PP: per protocol; ; RBC: red blood cell RCT: randomized clinical trial
Acknowledgements
The authors would like to thank to the patients their collaboration in the study.
Contributors
This article is part of a Doctoral Thesis of Miguel Angel Pico Picos on behalf of the Doctoral Program in Medicine. University of Gran Canaria. Canary Islands. (Spain).
All authors contributed to the design and development of the study. Likewise, all of them have collaborated in the preparation of the manuscript.
Disclosures
The authors declare none conflict of interest.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or non-profit sectors.
References
1. Sniecinski RM, Levy JH. Bleeding and management of coagulopathy. J Thorac Cardiovasc Surg. 2011; 142(3): 662-7.
2. Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogenic blood transfusion and the available strategies for their prevention. Blood. 2009; 113: 3406-17.
3. Robinson Y1, Hostmann A, Matenov A, et al. Erythropoiesis in multiply injured patients. J Trauma. 2006 Nov; 61(5): 1285-91.
4. Landi F, Russo A, Danese P, et al. Anemia status, hemoglobin concentration, and mortality in nursing home older residents. J Am Med Dir Assoc. 2007 Jun; 8(5): 322-7.
5. Madrazo-González Z, García-Barrasa A, Rodríguez-Lorenzo L, et al. Hierro intravenoso. Cir Esp. 2009; 86(4): 196-203.
6. Beris P, Muñoz M, García-Erce JA, et al. Perioperative anemia management: consensus statement on the role of intravenous iron. Br J Anaesth. 2008; 100(5): 599-604.
7. Minck S, Robinson K, Saxon B, et al. Patient blood management: the GP's guide. Aust Fam Physician. 2013; 42(5): 291-7.
8. Litton E, Xiao J, Ho KM. Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta-analysis of randomised clinical trials. BMJ. 2013 ;347: f4822.
9. Madi-Jebara SN, Sleilaty GS, Achouh PE, et al. Postoperative intravenous iron used alone or in combination with low-dose erythropoietin is not effective for correction of anemia after cardiac surgery. J Cardiothorac Vasc Anesth. 2004; 18(1): 59-63.
10. Johansson PI, Rasmussen AS, Thomsen LL. Intravenous iron isomaltoside 1000 (Monofer (®)) reduces postoperative anemia in preoperatively non-anaemic patients undergoing elective or subacute coronary artery bypass graft, valve replacement or a combination thereof: a randomized double-blind placebo-controlled clinical trial (the PROTECT trial). Vox Sang. 2015; 109(3): 257-66.
11. Chow SC, Chang M. Adaptive design methods in clinical trials, 2nd ed. Boca Raton: Chapman & Hall/CRC 2012.
12. Guyatt GH, Rennie D. The principle of intention to treat. In user´s guide to the medical literature. American medical association press. 2002.
13. Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann intern Med. 2001; 134: 657-62.
14. Food and drug Administration. Guideline for the format and content of the clinical and statistical sections of new drugs applications. FDA, US Department of Health and Human Services Rockville, MA, USA.1988.
15. Fisher LD, Dixon DO, Herson J, et al. Intention to treat in clinical trials. In: statistical Issues in Drug Research and Development (American Statistical Associations Group) Peace KE(ed) Marcel Dekker, New York. 1990: 331-50.
16. Gupta SK. Intention-to-treat concept: A review. Perspect Clin Res. 2011; 2(3): 109-12.
17. Karkouti K, McCluskey SA, Ghannam M, et al. Intravenous iron and recombinant erythropoietin for the treatment of postoperative anemia. Can J Anaesth. 2006; 53(1): 11-9.
18. Cladellas M, Farré N, Comín-Colet J, et al. Effects of preoperative intravenous erythropoietin plus iron on outcome in anemic patients after cardiac valve replacement Am J Cardiol. 2012; 110(7): 1021-6.
19. Banbury MK, Brizzio ME, Rajeswaran J, et al. Transfusion increases the risk of postoperative infection after cardiovascular surgery. J Am Coll Surg. 2006; 202(1): 131-8.
20. Yaffee DW, Smith DE 3rd, Ursomanno PA, et al. Management of blood transfusion in aortic valve surgery: impact of a blood conservation strategy. Ann Thorac Surg. 2014; 97(1): 95-101.
21. Theusinger OM, Leyvraz PF, Schanz U, et al. Treatment of iron deficiency anemia in orthopedic surgery with intravenous iron: efficacy and limits: a prospective study. Anesthesiology. 2007; 107(6): 923-7.
22. Cuenca J, García-Erce JA, Martínez AA, et al. Role of parenteral iron in the management of anemia in the elderly patient undergoing displaced subcapital hip fracture repair: preliminary data. Arch Orthop Trauma Surg. 2005; 125(5): 342-7.
23. García-Erce JA, Cuenca J, Muñoz M, et al. Perioperative stimulation of erythropoiesis with intravenous iron and erythropoietin reduces transfusion requirements in patients with hip fracture. A prospective observational study. Vox Sang. 2005; 88(4): 235-43.
24. Serrano-Trenas JA, Ugalde PF, Cabello LM, et al. Role of perioperative intravenous iron therapy in elderly hip fracture patients: a single-center randomized controlled trial. Transfusion. 2011; 51(1): 97-104.
25. Okuyama M, Ikeda K, Shibata T, et al. Preoperative iron supplementation and intraoperative transfusion during colorectal cancer surgery. Surg Today. 2005; 35(1): 36-40.
Received: April 25, 2022
Accepted: May 28, 2022
Published: May 30, 2022.
To cite this article : Ángel Pico-Picos M, Garrido-Martín P, Rodríguez-Fortúnez P, et al. A Randomized Clinical Trial about the Use of Intravenous Iron in Cardiac Surgery: Results of Three Analysis. British Journal of Heart Diseases. 2022;4(1): 182-187. doi: 10.31488/bjhd.116.
©2022 Ángel Pico-Picos M, et al.
